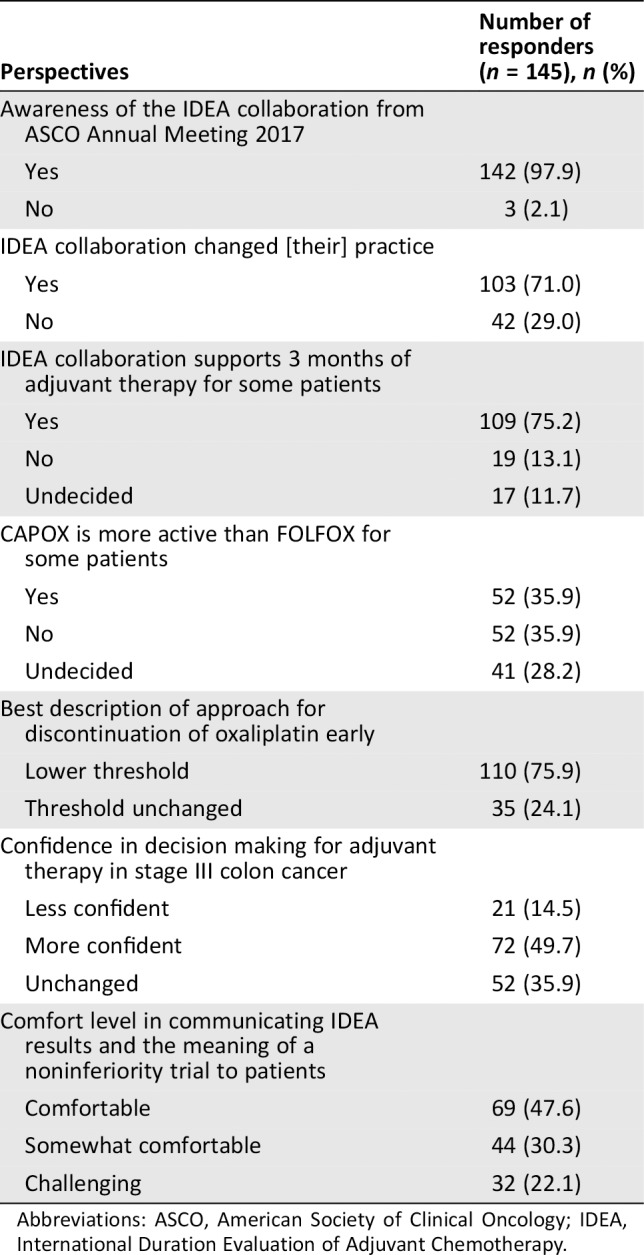
Table 2.
Survey responders’ perspectives on IDEA collaboration
| Perspectives | Number of responders (n = 145), n (%) |
|---|---|
| Awareness of the IDEA collaboration from ASCO Annual Meeting 2017 | |
| Yes | 142 (97.9) |
| No | 3 (2.1) |
| IDEA collaboration changed [their] practice | |
| Yes | 103 (71.0) |
| No | 42 (29.0) |
| IDEA collaboration supports 3 months of adjuvant therapy for some patients | |
| Yes | 109 (75.2) |
| No | 19 (13.1) |
| Undecided | 17 (11.7) |
| CAPOX is more active than FOLFOX for some patients | |
| Yes | 52 (35.9) |
| No | 52 (35.9) |
| Undecided | 41 (28.2) |
| Best description of approach for discontinuation of oxaliplatin early | |
| Lower threshold | 110 (75.9) |
| Threshold unchanged | 35 (24.1) |
| Confidence in decision making for adjuvant therapy in stage III colon cancer | |
| Less confident | 21 (14.5) |
| More confident | 72 (49.7) |
| Unchanged | 52 (35.9) |
| Comfort level in communicating IDEA results and the meaning of a noninferiority trial to patients | |
| Comfortable | 69 (47.6) |
| Somewhat comfortable | 44 (30.3) |
| Challenging | 32 (22.1) |
Abbreviations: ASCO, American Society of Clinical Oncology; IDEA, International Duration Evaluation of Adjuvant Chemotherapy.
