Table 2.
Randomized controlled trials currently ongoing or recently concluded (without available results) in lung cancer
| PI and Sponsor | Number | Title | Estimated participants | Primary outcome |
|---|---|---|---|---|
|
Chia‐Chin Lin
The University of Hong Kong |
NCT03482323 | Improving Survival in Lung Cancer Patients: A Randomized Controlled Trial of Aerobic Exercise and Tai‐Chi Interventions | 372 NSCLC | 1‐year OS |
|
Tora S. Solheim
Norwegian University of Science and Technology |
NCT02330926 | A Randomized, Open‐Label Trial of a Multimodal Intervention (Exercise, Nutrition and Anti‐Inflammatory Medication) Plus Standard Care Versus Standard Care Alone to Prevent/Attenuate Cachexia in Advanced Cancer Patients Undergoing Chemotherapy [MENAC trial] | 240 LC and PDAC | Body weight |
|
Kathleen Lyons
Dartmouth‐Hitchcock Medical Center |
NCT03500393 | A Remotely Supervised Exercise Program for Lung Cancer Patients Undergoing Chemo‐Radiation (REM) | 50 LC | Recruitment and retention |
|
Lee W. Jones
Memorial Sloan Kettering Cancer Center |
NCT01068210 | Lung Cancer Exercise Training Study: A Randomized Trial of Aerobic Training, Resistance Training, or Both in Lung Cancer Patients | 160 LC | VO2peak |
|
Sandy Jack
University Hospitals Southampton NHS Foundation Trust |
NCT03334071 | Exercise Regimens Before and During Advanced Cancer therapy: A Pilot Study to Investigate Improvements in Physical Fitness with Exercise Training Programme Before and During Chemotherapy in Advanced Lung Cancer Patients | 100 NSCLC | Adherence to exercise training program and adverse events |
|
Morten Quist
Rigshospitalet, Denmark |
NCT03066271 | PRIME ‐ Pre Radiotherapy Daily Exercise Training in Non‐Small Cell Lung Cancer | 40 NSCLC | VO2peak |
|
Paul LaStayo
University of Utah |
NCT03306992 | A Phase III Randomized Study Comparing the Effects of a Personalized Exercise Program (PEP) Against No Intervention in Patients with Stage I–IIIa Primary Non‐Small Cell Lung Cancer or Secondary Lung Cancer Undergoing Surgical Resection | 200 LC (primary or secondary) | 6MWT |
|
Amy Hoffman
University of Nebraska |
NCT03724331 | Understanding the Post‐Surgical Non‐Small Cell Lung Cancer Patient's Symptom Experience | 279 NSCLC | Fatigue |
|
Brett Bade
Yale University |
NCT03352245 | Assessing the Feasibility of a Patient‐centered Activity Regimen in Patients with Advanced Stage Lung Cancer | 40 NSCLC | Steps count and adherence to recommendations |
|
Marta Kramer Mikkelsen
Herlev and Gentofte Hospital |
NCT03411200 | Engaging the Older Cancer Patient; Patient Activation Through Counseling, Exercise and Mobilization ‐ Pancreatic, Biliary Tract, and Lung Cancer (PACE‐Mobil‐PBL) ‐ A Randomized Controlled Trial | 100 NSCLC, PDAC, biliary cancer | Lower body strength |
|
Jesper Holst Pedersen
Rigshospitalet, Denmark |
NCT02439073 | Postoperative Rehabilitation in Operation for LUng CAncer (PROLUCA) ‐ A Randomized Clinical Trial with Blinded Effect Evaluation | 235 LC | VO2peak |
|
Elisabeth Edvardsen
Oslo University Hospital |
NCT01748981 | Cardiorespiratory Fitness and Effect of Training After Lung Cancer Surgery. A Randomized Controlled Trial | 80 NSCLC | VO2peak |
|
Grandes Gonzalo
Basque Health Service |
NCT01786122 | Physical Exercise to Improve the Quality of Life in Cancer Patients During Treatment Process: EFFICANCER Study | 250 NSCLC, GI, and BC | QoL |
|
Liu Jui Fang
Chang Gung Memorial Hospital |
NCT02757092 | The Impacts of Pulmonary Rehabilitation Therapy on Patients After Thoracic Surgery | 120 mixed | Pulmonary complication |
|
Sara Tenconi
Arcispedale Santa Maria Nuova‐IRCCS |
NCT02405273 | Effects of Early Pulmonary Rehabilitation and Long‐Term Exercise on Functioning, Quality of Life and Postoperative Outcome in Lung Cancer Patients | 140 NSCLC | 6MWT |
|
Miklos Pless
Kantonsspital Winterthur KSW |
NCT02585362 | Influence of a Specially Formulated Whey Protein Supplement in Combination with Physical Exercise and Nutrition Program on Physical Performance in Cancer Outpatients | 88 mixed | Physical performance |
|
Ling Xu
Shanghai University of Traditional Chinese Medicine |
NCT03244605 | Clinical Study on the Effect of Comprehensive Rehabilitation Program on Quality of Life and Long‐Term Survival in Postoperative Non‐Small Cell Lung Cancer Patients | 236 NSCLC | QoL |
|
Alice Ryan
University of Maryland |
NCT02991677 | Exercise Effect on Chemotherapy‐Induced Neuropathic Pain, Peripheral Nerve Fibers | 60 mixed | Pain |
|
Young Sik Park
Seoul National University Hospital |
NCT02121379 | Randomized Clinical Trial of 8 Weeks Pulmonary Rehabilitation in Advanced Stage Lung Cancer Patients with COPD During Cytotoxic Chemotherapy | 40 LC | VO2peak |
|
Joseph A. Greer
Massachusetts General Hospital |
NCT03089125 | Brief Behavioral Intervention for Dyspnea in Patients with Advanced Lung Cancer | 200 LC | Dyspnea |
|
Oscar Gerardo Arrieta Rodríguez
Instituto Nacional de Cancerologia de Mexico |
NCT02978521 | Effect of a Pulmonary Rehabilitation Program on Skeletal Muscle Mass, Pulmonary Function, Inflammatory Response and Overall Survival on Patients Diagnosed with Non‐Small‐Cell Advanced Cancer | 94 NSCLC | Pulmonary function |
|
Ling Xu
Shanghai University of Traditional Chinese Medicine |
NCT03372694 | Efficacy Study of Comprehensive Rehabilitation Program Plus Chemotherapy in Postoperative NSCLC Patients | 354 NSCLC | QoL |
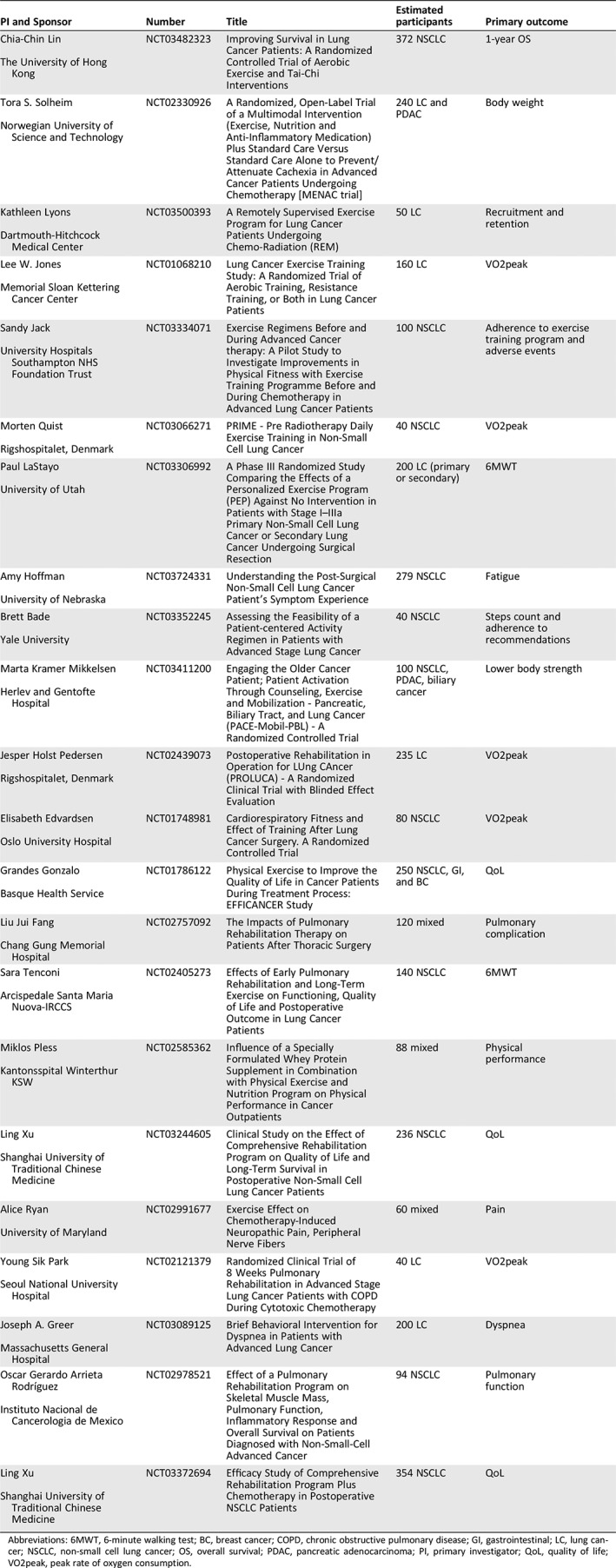
Abbreviations: 6MWT, 6‐minute walking test; BC, breast cancer; COPD, chronic obstructive pulmonary disease; GI, gastrointestinal; LC, lung cancer; NSCLC, non‐small cell lung cancer; OS, overall survival; PDAC, pancreatic adenocarcinoma; PI, primary investigator; QoL, quality of life; VO2peak, peak rate of oxygen consumption.
