Figure 1.
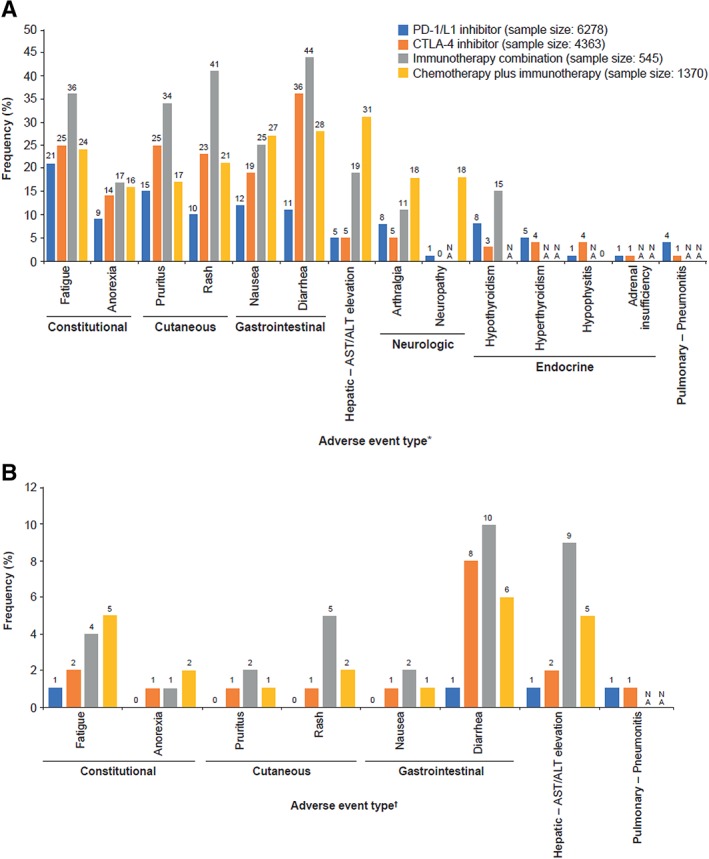
Frequency of (A) any grade and (B) grade 3/4 adverse events in clinical trials of PD‐1/L1 inhibitors, CTLA‐4 inhibitors, immunotherapy combinations, and chemotherapy plus immunotherapy combinations 70. *, Myositis and mucositis not included because these adverse events were NA for all groups except for PD‐1/L1 inhibitor. †, Adverse events from panel A with grade 3/4 frequency of 0 or NA in ≥3 groups were excluded. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTLA‐4, cytotoxic T‐lymphocyte–associated antigen 4; NA, not available; PD‐1, programmed death‐1; PD‐L1, PD‐1 ligand.
