Table 1.
Patient demographics and baseline characteristics among patients in the T‐B group or the T group
| Characteristic | T‐B group (n = 21)a | T group (n = 36)a |
|---|---|---|
| Sex, n | ||
| Male | 13 | 16 |
| Female | 8 | 20 |
| Age, median | 67.0 (50.0–74.0) | 67.5 (59.8–71.2) |
| Height, cm | 164.0 (158.0–168.0) | 159.5 (152.0–164.0) |
| Body weight, kg | 61.8 (55.8–68.4) | 54.9 (47.0–60.5) |
| Body mass index | 23.1 (22.4–23.9) | 21.2 (19.7–23.6) |
| Albumin, mg/dL | 3.9 (3.6–4.1) | 3.7 (3.1–3.9) |
| Aspartate aminotransferase, IU/L | 24.0 (22.0–38.0) | 32.0 (21.0–52.0) |
| Alanine aminotransferase, IU/L | 17.0 (14.0–29.0) | 17.5 (12.8–26.8) |
| Serum creatinine, mg/dL | 0.6 (0.6–0.9) | 0.6 (0.5–0.7) |
| Total bilirubin, mg/dL | 0.6 (0.5–0.8) | 0.7 (0.5–1.0) |
| CRP, mg/dL | 0.4 (0.3–1.8) | 1.7 (0.3–4.0) |
| Neutrophils, /L | 3,670.0 (3,030.0–4,500.0) | 4,515.0 (3,607.8–6,250.0) |
| Lymphocytes, /L | 1,564.0 (1,269.0–1,772.0) | 1,425.0 (980.5–2,102.2) |
| White blood cells, /L | 5,900.0 (5,170.0–7,020.0) | 7,640.0 (5,645.0–9,020.0) |
| Hemoglobin, g/dL | 12.7 (12.1–13.8) | 11.4 (10.3–12.7) |
| Platelets, 104/L | 21.5 (17.5–28.1) | 22.1 (18.1–26.4) |
| mGPS, n | ||
| 0 | 16 | 11 |
| 1 | 4 | 11 |
| 2 | 1 | 9 |
| NLR | 2.5 (1.8–3.4) | 3.2 (2.1–4.6) |
| CEA, U/mL | 37.2 (22.5–98.2) | 101.8 (35.9–260.1) |
| CA19‐9, U/mL | 122.0 (32.0–330.0) | 182.0 (41.2–503.7) |
| Time from start of first‐line chemotherapy, days | 646.0 (534.0–820.0) | 693.0 (410.5–503.7) |
| Primary site, n | ||
| Colon | 17 | 34 |
| Rectal | 4 | 2 |
| Primary site, n | ||
| Right | 14 | 14 |
| Left | 7 | 22 |
| Number of metastatic organs or sites, n | ||
| 1 | 7 | 12 |
| ≥2 | 14 | 24 |
| Metastatic organ, n (%) | ||
| Liver | 15 (71) | 25 (69) |
| Lung | 15 (71) | 28 (78) |
| Lymph nodes | 5 (24) | 11 (31) |
| Peritoneum | 4 (19) | 12 (33) |
| Recurrent/advanced | ||
| Recurrent | 4 | 13 |
| Advanced | 17 | 23 |
| KRAS exon 2 status, n | ||
| Wild type | 10 | 16 |
| Mutant | 11 | 20 |
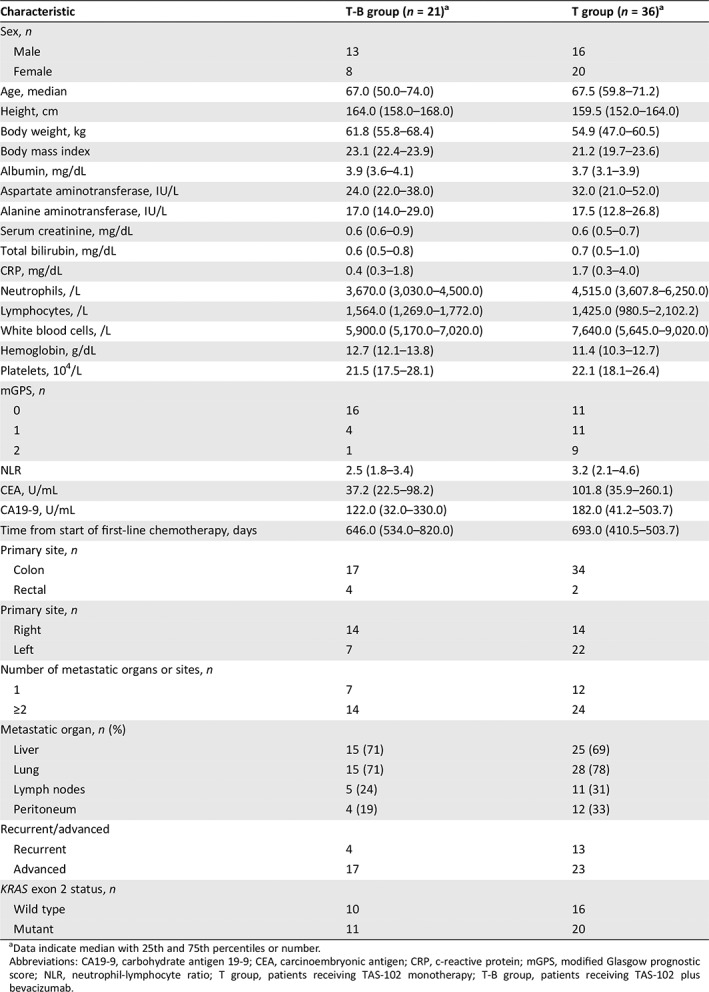
Data indicate median with 25th and 75th percentiles or number.
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; CRP, c‐reactive protein; mGPS, modified Glasgow prognostic score; NLR, neutrophil‐lymphocyte ratio; T group, patients receiving TAS‐102 monotherapy; T‐B group, patients receiving TAS‐102 plus bevacizumab.
