Abstract
Background
The expression of vascular endothelial growth factor (VEGF)-A/ VAGF receptors (VEGFRs) signaling plays a pivotal role in the tumor angiogenesis and the development of the immunosuppressive tumor microenvironment in glioblastomas. We have previously conducted exploratory clinical studies investigating VEGFRs peptide vaccination with and without multiple glioma oncoantigens in patients with recurrent high-grade gliomas. Recently, an exploratory clinical investigation of VEGFRs peptide vaccination was conducted in patients with progressive neurofibromatosis type 2. Those studies suggested that cytotoxic T lymphocytes (CTLs) induced by the vaccination can directly kill a wide variety of cells associated with tumor growth, including tumor vessels, tumor cells, and immunosuppressive cells expressing VEGFR1 and/or 2. In the present study, synergistic activity of the combination of VEGFRs peptide vaccination with chemotherapy was evaluated.
Methods
We performed the first clinical trial to assess VEGFR1 and 2 vaccination along with temozolomide (TMZ) -based chemoradiotherapy for the patients with primary glioblastomas. Furthermore, histopathological changes after the vaccination were evaluated using paired pre- and post- vaccination specimens.
Results
The disappearance of radiographically enhanced lesion was observed in 2 patients after the vaccination, including one in which the methylation of the O6-methylguanine-DNA methyltransferase (MGMT) promoter was not observed. The histopathological findings of pre- and post-vaccination specimens demonstrated that tumor vessels showed negative or slight VEGFRs expressions after the vaccination and most endothelial cells were covered with PDGFR-β-positive pericytes. Notably, CTLs induced by VEGFRs peptide vaccination attacked not only tumor vessels but also tumor cells and regulatory T cells expressing VEGFRs even in recurrent tumors.
Conclusions
VEGFR1 and 2 vaccination may have a preliminary synergistic effect when administered with TMZ. The limitation of the present study was the paucity of the number of the samples. Further studies involving more patients are warranted to confirm the findings of this study.
Trial registration
This study was registered as UMIN000013381 (University Hospital Medical Information Network-Clinical Trial Registry: UMIN-CTR) on 5 March, 2014 and with the Japan Registry of Clinical Trials (jRCT) as jRCTs031180170 on 1 March, 2019.
Keywords: VEGFR, Peptide vaccine, Glioblastoma, Bevacizumab
Background
Angiogenic factors are important for the growth of malignant tumors, and vascular endothelial growth factor (VEGF)-A/ VEGF receptors (VEGFRs) signaling is the most potent [1]. Glioblastoma is a highly malignant tumor that exhibits extensive vascularity. The expression of VEGF-A/ VEGFRs is strongly upregulated in glioblastoma, and the expression degree correlates with the grade of malignancy and prognosis in malignant glioma [2, 3]. It has been reported that both VEGFR1 and VEGFR2 are expressed on not only vascular endothelial cells, but also tumor cells [4]. VEGFR1 signaling is critical for tumor growth [5]. VEGFR2 plays an important role in the proliferation of tumor stem cells [6]. Furthermore, VEGF/VEGFR signaling plays a pivotal role in the development of the immunosuppressive tumor microenvironment in glioblastomas [7].
Therefore, VEGF-A and VEGFRs targeted anti-angiogenic therapies have been previously used in glioblastomas. Bevacizumab, which targets circulating VEGF-A, and multikinase inhibitor, such as cediranib, sunitinib, and sorafenib, were associated with favorable event-free survival in patients with glioblastomas [8–12]. In addition, combinational therapy with anti-angiogenic therapy and chemotherapy has been an attractive treatment strategy in glioblastomas, because anti-angiogenic therapy induces a functional normalization of the tumor vasculature, increasing tumor cell exposure, and enhancing the activity of co-administered chemoradiotherapies [13]. The combinational therapy involving sorafenib plus daily temozolomide (TMZ), which is the primary chemotherapy used globally for glioblastoma treatment [14], was used for the recurrent glioblastomas, indicating that it is feasible, safe, and has some effect on patients [15].
Cancer immunotherapy has become the fourth preferred modality of cancer treatment after surgery and chemoradiotherapy. Peptide vaccination is an immunotherapy that aims to activate cytotoxic T lymphocytes (CTLs) in patients by inoculating antigen peptides. We have previously conducted exploratory clinical studies investigating VEGFRs peptide vaccination with and without multiple glioma oncoantigens in patients with recurrent high-grade gliomas [16, 17]. Recently, VEGFRs peptide vaccine was used in patients with progressive neurofibromatosis type 2 (NF2) [18]. In the study, the number of Foxp3-positive regulatory T cells (Tregs) decreased after the vaccination, suggesting that the CTLs induced by the vaccination can directly kill a wide variety of cells associated with tumor growth, including tumor vessels, tumor cells, and Tregs expressing VEGFR1 and/or VEGFR2. Furthermore, the combinational usage of chemotherapy and immunotherapy is also effective with synergistic activity [19], because chemotherapy suppressed immunosuppressive T cells and immunotherapy sustains the proliferation of potential effector immune cells [20, 21].
Based on these backgrounds, in this trial, VEGFR1 and 2 vaccine was used with TMZ-based chemoradiotherapy for the patients with primary glioblastomas [14]. VEGFRs peptide vaccination might have both the advantages of anti-angiogenic therapy and immunotherapy. In addition, we successfully evaluated the histopathological changes after the VEGFR1 and 2 vaccination using paired pre- and post-vaccination patient-derived specimens, proving the synergic effects when administered with chemotherapy.
Methods
Trial overview
The present study was an exploratory phase I and II clinical trial to assess the feasibility and effectiveness of VEGFR1 and 2 peptide vaccination in primary glioblastoma. All protocols were approved by the Keio University School of Medicine Ethics Committee (reference number: 20130461), and conducted in accordance with the Helsinki declaration on experimentation on human subjects. The trial was registered at UMIN (UMIN000023565) and jRCT (jRCTs031180170). The authors affirm that human research participants provided informed consent to participate in the study and for publication of their data.
Patient eligibility
Patients with high-grade glioma (WHO grade III or IV) after standard treatment (surgical removal + radiotherapy (RT) concomitant with TMZ [14]) were enrolled in this clinical study at the Department of Neurosurgery, Keio University School of Medicine. Patients also had to show positive for the genomic DNA typing test for HLA-A*2402 (HLA Laboratory, Kyoto, Japan). Details of the inclusion and exclusion criteria are provided in Table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
• Histological diagnosis of high-grade glioma (WHO grade III or IV) • Announcement of a diagnosis • Positive genomic DNA typing test for HLA-A*2402 (HLA Laboratory, Kyoto, Japan) • Age between 16 and 79 • Eastern Cooperative Oncology (ECOG) performance status (PS) 0–2 • Completion of standard treatment (surgical removal + radiotherapy concomitant with temozolomide) • No prior surgery, irradiation, or chemotherapy 4 weeks before entry to the study • No uncontrollable pleural, peritoneal or cardiac effusion • Life expectancy > 3 months • Written informed consents are obtained. Lab values prior to vaccine • Neutrophil count ≥1000/mm3 • Platelet count ≥500,00/mm3 • Hemoglobin level ≥ 8.0 g/dl, a • Aspartate aminotransferase and alanine aminotransferase ≤4.0x the institutional normal upper limits • Total bilirubin ≤1.5x • Creatinine ≤2.0 mg/dL • No uncontrollable pleural, peritoneal or cardiac effusion |
• The presence of uncontrollable severe infectious diseases • Adverse event of National Cancer Institute - Common Toxicity Criteria (NCI-CTC) grade 3 or 4 • Unable to take anything orally over 24 h • Other uncontrolled malignant diseases • Myeloproliferative diseases • After allogeneic hematopoietic stem cell transplantation • Active autoimmune diseases • Severe drug allergy • Concurrent treatment with steroids or immunosuppressive agents • Pregnant women or patients who planned to become pregnant during the study period • Psychiatric disorders • Unhealed wound • Decision of unsuitability by the principal investigator or the physician in charge. |
Peptides
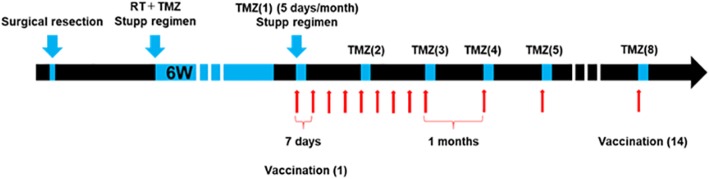
Good manufacturing practice (GMP)-graded VEGFR1-A24–1084 peptide (SYGVLLWEIF) and VEGFR2-A24–169 peptide (RFVPDGNRI) were synthesized by BCN Peptides S.A. according to a standard solid-phase synthesis method and purified by reversed-phase high-performance liquid chromatography (HPLC). The purity (> 95%) and the identity of the peptides were determined by analytical HPLC and mass spectrometry, respectively. VEGFR1-A24–1084 and VEGFR2-A24–169 peptide (2 mg of each) were emulsified together with 1 ml of incomplete Freund’s adjuvant (Montanide ISA-51 VG, SEPPIC, Paris) and injected subcutaneously at infra-axillary and inguinal lymph nodes eight times every week and then six times monthly (a total of 14 times). Vaccination was synchronized with adjuvant TMZ [14] (Fig. 1). The period of this study was 12 months starting after the 1st vaccination.
Fig. 1.
The Protocol for this clinical trial. The scheme of the Protocol for this clinical trial is shown. VEGFR1 and R2 peptide were injected eight times every week and then six times monthly (a total of 14 times). Vaccination was synchronized with adjuvant TMZ
Outcomes and assessments
The primary endpoints were safety and clinical efficacy of the vaccination [median overall survival (OS) time]. OS was defined as the interval from the date of commencement of treatment to the date of death. The secondary endpoints were radiographical and immunological responses. Toxicities were assessed with the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE ver4.0) at each visit. To evaluate the clinical response, magnetic resonance imaging (MRI) were performed within 2 weeks before the first cycle, and then after 8, 12 and 14 cycles (3, 6, and 12 months). Radiographical response was evaluated by Response Assessment in Neuro-Oncology (RANO) and immunotherapy RANO (iRANO) using gadolinium (Gd) -enhanced T1 weighted images and fluid attenuated IR (FLAIR) on the basis of the appearance of the pretreatment MRI [22, 23]. Peptide-specific immunological responses were analyzed by Enzyme-Linked ImmunoSpot (ELISPOT) assay (for details, see the Methods section in Additional file 3) [16, 24].
Molecular-genetic analysis
Chromosomal number aberrations (CNAs) were assessed by metaphase comparative genomic hybridization, as previously described [25]. Mutation of the isocitrate dehydrogenase (IDH)1 gene, and O6-methylguanine DNA methyltransferase (MGMT) promoter methylation, were also analyzed as previously described [16].
Immunohistochemical analysis
Histopathological analyses were performed on 4-μm sections of formalin-fixed, paraffin-embedded tissues of paired pre- and post-vaccination obtained from Case 2. The expression of VEGF-A, VEGFR1, VEGFR2, CD34 (endothelial cell marker), PDGFR-β (pericyte marker), programmed cell death-1 (PD-1) and programmed cell death ligand-1 (PD-L1) (immune-checkpoint molecules), CD4 (helper T-cell marker), CD8 (CTL marker), Foxp3 (Treg marker), CD163 (tumor-associated macrophage [TAM] marker), nestin (neural stem/ progenitor cell marker), and cleaved caspase 3 (apoptosis marker) were analyzed [26, 27].
Immunofluorescence staining for VEGFR1, VEGFR2, and PDGFR-β expressions or VEGFR1, VEGFR2, and cleaved caspase 3, CD34, VEGFR1 and cleaved caspase 3, or Foxp3 and cleaved caspase 3 expressions was performed (for details, see the Methods section in Additional file 3).
RNA extraction, cDNA synthesis, and quantitative real-time PCR
For quantitative real-time PCR (qPCR) of VEGF-A, VEGFR1, VEGFR2 and Foxp3, RNA was isolated from 10-μm sections of formalin-fixed, paraffin-embedded tissue using the “NucleoSpin total RNA FFPE XS” Kit (Macherey-Nagel) (for details, see the Methods section in Additional file 3) [28].
Statistical analysis
PFS was defined as the date elapsed between treatment initiation and tumor progression. OS and PFS were analyzed based on the Kaplan-Meier test. All statistical analyses were performed with IBM SPSS statistics. Differences were considered to be statistically significant when p < 0.05.
Results
Patient characteristics
Four patients with primary glioblastoma were enrolled in this study (39–75 years old, two males and two females) between September 2014 and March 2018. All patients received 14 cycles of VEGFRs peptide vaccination (Table 2). All cases were IDH1-R132H wild-type and MGMT promoter methylation appeared in Case 2 and 4. 1,3-bis-Chloroethyl-1-Nitrosourea (BCNU) wafer was used in the surgical treatment of Case 2 and 3. The data of CGH analysis are summarized in Table 2.
Table 2.
Patients’ Characteristics
| Case | Age | PS | MIB-1 index | IDH1 Mutation | MGMT Methylation | CGH | Surgical removal | Radiation | TMZ cycles | Other treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 75 | 1 | 40.1 | wild | – | + 7p15.2-qter, − 10, −15q, (−16qcen-13) | GTR | 40Gy/15fr | 22 | – |
| 2 | 39 | 1 | 11.1 | wild | + | +1pter-34.1, −9pter-21, − 10q21.1-ter, +13q12.2–31, +17p12-q21.1, −18q23, −21q, +22qcen-13.1 | GTR | 60Gy/30fr | 31 | BCNU wafer |
| 3 | 52 | 0 | 30 | wild | – | -1pter-36.1, + 7, −9pter-21, − 10 | GTR | 60Gy/30fr | 14 | BCNU wafer |
| 4 | 50 | 0 | 50 | wild | + | + 7, − 10, +12q15, +13q14.3–33 | GTR | 60Gy/30fr | 14 | – |
BCNU bis-chloroethylnitrosourea, CGH comparative genomic hybridization, GTR gross total resection, IDH isocitrate dehydrogenase, MGMT O6 methylguanine DNA methyltransferase, PS performance status, TMZ temozolomide
Adverse events
No major toxicity (grade 3 and 4) was found in this study. During this vaccination, Case 4 developed grade 1 local skin reaction at the injection sites with induration, redness, and swelling. No patients developed ulcers at the injection sites. No delayed wound healing or gastrointestinal bleeding were seen either. No other adverse events, such as arterial and venous thromboembolism, hypertension, and proteinuria, which were reported in the clinical study of bevacizumab, were detected.
Clinical response
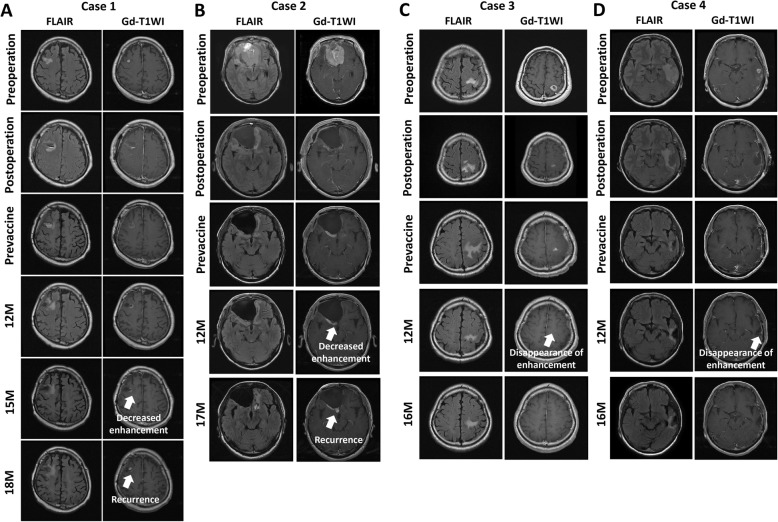
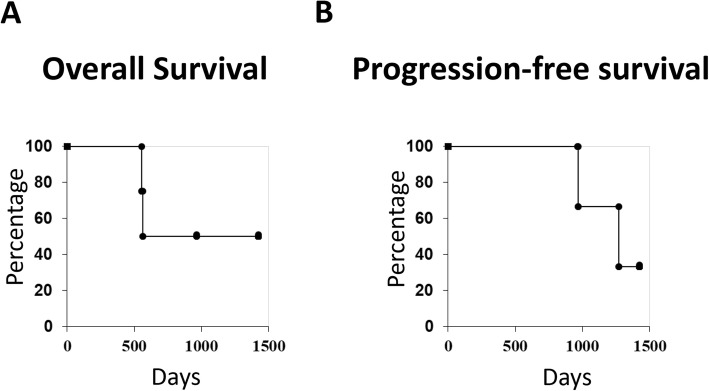
Case 3 and 4 achieved complete response (CR). MGMT methylation was not detected in Case 3 (Fig. 2c, d). Although Gd-enhanced lesions were temporarily decreased in Case 1, and 2, Case 1 revealed progressive disease (PD) 10 months after the last vaccination and 9 months in Case 2 (Fig. 2a, b). In Case 2, the recurrent enhanced lesion was surgically removed again. The Kaplan–Meier curves for OS and PFS in four patients are shown in Fig. 3, respectively. At the time of analysis, Case 3 and 4 still showed CR (1425 and 962 days). Case 1 and 2 had already died (OS: Case 1, 967 days; Case 2, 1272 days).
Fig. 2.
Radiographic images of enrolled patients. Radiographic images of Case 1(a), Case 2 (b), Case 3 (c), Case 4 (d). a The enhanced lesion was decreased 15 months, and recurrent lesion was observed at the removal site 18 months after the first vaccination. b The enhanced lesion was decreased 12 months, and recurrent lesion was observed at the removal site 17 months after the first vaccination. c The enhanced lesion disappeared 12 months after the first vaccination. d The enhancement lesion disappeared 12 months after the first vaccination
Fig. 3.
Clinical course of enrolled patients. a Overall survival of four patients. At the time of analysis, Case 3 and 4 still had CR. b Progression-free survival of four patients
CD8+ T-cell response
In Case 3 and 4, CTLs specific for both VEGFR1 and 2 were induced after vaccination. Immunological monitoring could not be performed for Case 1 and 2, as the samples were lost because of a deep freezer fault (Table 3).
Table 3.
Clinical results
| Case No. | DTH | Vac cycles | Toxicity | ELISPOT (CTL) | CTL induction | PFS (days) | OS (days) | Evaluation after 12 M | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| timing | R1 | R2 | R1 | R2 | |||||||
| 1 | – | 14 | – | Before | N/A | N/A | N/A | N/A | 554 | 967 | PR |
| After | N/A | N/A | |||||||||
| 2 | – | 14 | – | Before | N/A | N/A | N/A | N/A | 562 | 1272 | SD |
| After | N/A | N/A | |||||||||
| 3 | – | 14 | – | Before | – | + | + | + | – |
Still survive (1425) |
CR |
| After | + | 3+ | |||||||||
| 4 | + | 14 | – | Before | – | N/A | + | + | – | Still survive (962) | CR |
| After | 3+ | 2+ | |||||||||
CTL cytotoxic T lymphocyte, DTH delayed type hypersensitivity, M month, N/A not available, OS overall survival, PD progressive disease, PFS progression-free survival, PR partial response, R vascular endothelial growth factor receptor, SD stable disease, Vac vaccination
Histopathological analysis
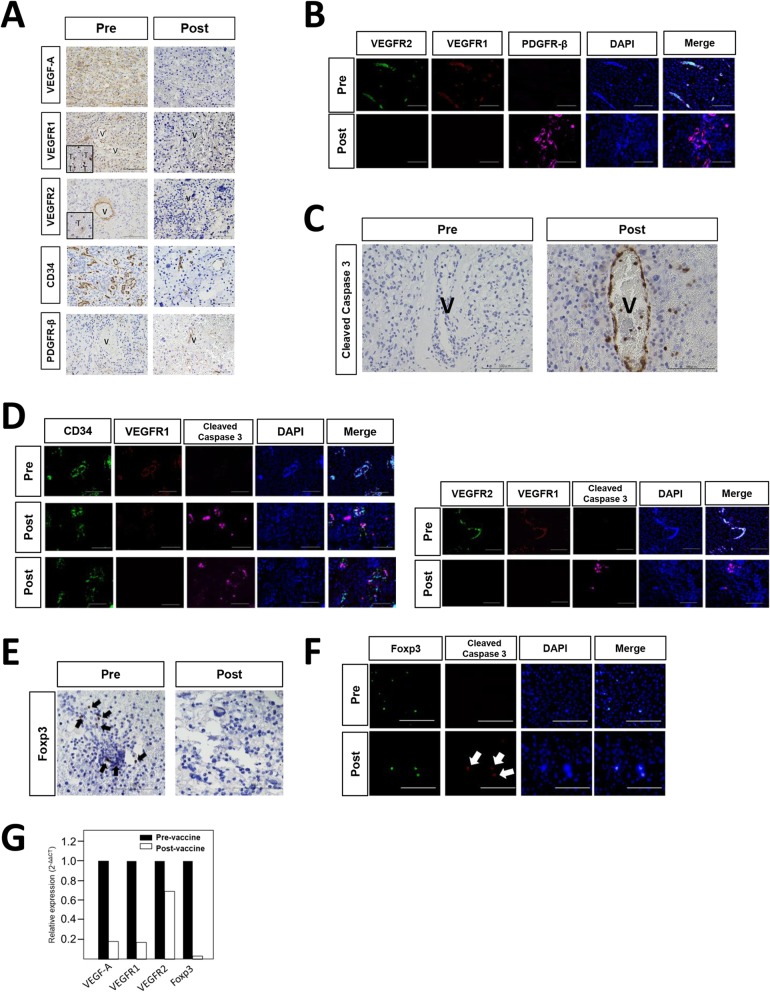
The histological changes using pre- and post-vaccination glioblastoma specimens could be analyzed in Case 2. The analysis of endothelial cells stained by CD34 demonstrated that vessel diameter was smaller, and microvessel density (MVD) was lower after vaccination. Most tumor vessels exhibited strong VEGFRs expressions without PDGFR-β positive pericytes before vaccination. In contrast, tumor vessels after vaccination showed negative or slight expression of VEGFR1 and 2, and most endothelial cells were covered with PDGFR-β positive pericytes (Fig. 4a, b). Tumor cells with VEGFR1 or R2 expression were observed in the pre-vaccination tumor (Fig. 4a). VEGF-A expression after vaccination was also decreased compared with that before vaccination (Fig. 4a). There were fewer Foxp3-positive cells in the post-vaccination tumor compared with that of pre-vaccination (Fig. 4e). qPCR analysis revealed that the mean relative expressions of VEGF-A, VEGFRs, and Foxp3 genes in the post-vaccination tumors were lower than that of the pre-vaccination tumor (Fig. 4g). More number of cleaved caspase 3 positive cells were detected in post-vaccination tumor than in pre-vaccination tumor. In the post-vaccination tumor, the expression of cleaved caspase 3 was co-localized in endothelial cells with CD34-positive staining and Foxp3-positive cells (Fig. 4c, d, e, f). In contrast, the number of CD163, CD8, and CD4-positive cells did not change after vaccination. Immune checkpoint molecules, such as PD-1/PD-L1 and, a marker of glioma stem cell-like phenotype, such as nestin did not change either after vaccination (Additional file 1: Figure S1 A,B,C).
Fig. 4.
Histopathological images of the paired pre- and post-vaccination. a Histopathological analysis of VEGF-A, VEGFR1, VEGFR2, CD34 and PDGFR-β expressions in the tumor of pre- and post- vaccination. A square means tumor cells with positive VEGFR1 or VEGFR2 staining (T: tumor cell, V: vessel; original magnification, × 40; magnification bar, 100 μm). b Immunofluorescent analysis of VEGFR1, VEGFR2 and PDGFR-β expressions in tumor vessels in pre- and post- vaccination (original magnification, × 40; magnification bar, 100 μm). c Histopathological analysis of cleaved caspase3 expression on the tumor vessel of pre- and post- vaccination. (V: vessel; original magnification, × 40; magnification bar, 100 μm). d Immunofluorescent analysis of CD34, VEGFR1, and cleaved caspase3 expressions in the tumor of pre- and post- vaccination. Expression of cleaved caspase 3 was detected on the endothelial cells with slight VEGFR1 expression or without VEGFR1 expression (original magnification, × 40; magnification bar, 100 μm). e Histopathological analysis of Foxp3 expression in the tumor of pre- and post- vaccination (original magnification, × 40; magnification bar, 100 μm; black arrows, positive cells). f Immunofluorescent analysis of Foxp3 and cleaved caspase3 expressions in the tumor of pre- and post- vaccination (original magnification, × 40; magnification bar, 100 μm; white arrow, positive cells). g Relative gene expression of VEGF-A, VEGFR1, VEGFR2 and Foxp3 in the tumors of pre- and post- vaccination
Discussion
VEGF /VEGFRs signaling plays a pivotal role in the tumor angiogenesis and the development of the immunosuppressive tumor microenvironment in glioblastomas by inhibiting the maturation of dendritic cells (DCs) and stimulating the proliferation of Tregs, TAMs, and myeloid-derived suppressor cells (MDSCs) with VEGFRs expressions [7, 29–32]. Therefore, anti-angiogenic therapy targeting VEGF and/or VEGFRs, including VEGFRs peptide vaccination, has not only anti-angiogenic effects, but also immune-supportive effects [19, 26]. In addition, VEGFRs peptide vaccination has the advantages of immunotherapy. CTLs induced by the vaccination may persist in the long-term. In the present study, the synergistic activity of the combinational usage of VEGFRs peptide vaccination and TMZ-based chemotherapy was investigated for the patients with primary glioblastomas.
In this study, the disappearance of a radiographically enhanced lesion in the patient with unmethylated MGMT promoter was suggestive. The histopathological changes after the VEGFRs vaccination using paired pre- and post-vaccination specimens demonstrated that VEGFR1 and 2 peptide vaccination induced the normalization of vascular structure with decreased VEGFR1 and 2 expressions, and the reduction of MVD in the recurrent tumor after vaccination. We have previously reported the histopathological changes after the administration of bevacizumab (anti-VEGF-A monoclonal antibody) using actual human glioblastoma specimens resected in 3 different settings: glioblastomas before any treatment; glioblastomas resected following bevacizumab therapy; and recurrent glioblastomas after long-term bevacizumab therapy [26, 27, 33]. In these previous studies, the expressions of VEGFR1 and 2 were upregulated in recurrent glioblastomas after long-term bevacizumab therapy [33]. The present histopathological results might suggest that memory CTL induced by VEGFRs peptide vaccination may overcome the problems of anti-angiogenic molecular targeting agents, which include apparent drug resistance and rebound upregulated VEGF-A/VEGFRs signaling [34]. However, peptide-based vaccination results in the induction of T cell exhaustion. The transient upregulation of PD-1 during T-cell activation and its maintenance on chronically stimulated exhausted T cells enable PD-1 to negatively regulate T-cell function [35, 36]. Therefore, Immune checkpoint inhibition may exert synergic effects when administered with this type of CTL-mediated antitumor immunotherapy. In the future, we will reveal the difference in the target of inhibition, VEGF compared with VEGFRs using these valuable human tumor specimens.
Furthermore, the combinational usage of chemotherapy and immunotherapy was reported to be effective with synergistic activity [19–21]. The combination of TMZ and immunotherapy with fusions of dendritic cells (DCs) and glioma cells safely induced anti-tumor effects in patients with glioblastomas [37]. However, the Tregs population increases rapidly, known as the rebound phenomenon, during long-term TMZ therapy for glioblastomas [38]. Importantly, the present histopathological finding of cleaved caspase 3 also proved that VEGFR1 and 2 peptide vaccination could target wide variety of cells associated with tumor growth, such as vascular endothelial cells, tumor cells, and Foxp3 + Tregs expressing VEGFR1 and/or VEGFR2, which was considered as one of the rationales behind using VEGFRs peptide vaccination with TMZ. Previous study also demonstrated that VEGFR2-targeting treatment has the possibility of selectively killing Tregs, because Foxp3(+) Tregs express VEGFR2 [39, 40], which was compatible with the present results.
Although immunotherapy has become an increasingly available and vital cancer treatment option, it has some disadvantages. Immunotherapy’s potential side effects result from an overstimulated or misdirected immune response. However, fewer severe side effects were reported in the clinical setting. The clinical trials with peptide-based vaccine therapy using VEGFR-derived epitopes have been previously conducted for the patients with advanced gastrointestinal cancers and renal cell cancer, wherein the treatment exhibited the safety (Additional file 2: Table S1) [20, 41–47]. Chemotherapy attacks all rapidly-dividing cells within the body, effectively targeting fast-growing tumors. In contrast, immunotherapy takes longer time to work compared with other treatments [48].
The present study might demonstrate that the preliminary safety and immunogenicity of this approach. VEGFR1 and 2-specific CTLs inductions were detected under treatment with TMZ. In addition, paired pre- and post-vaccination specimens suggested that VEGFR1 and 2 peptide vaccination may possibly enhance the effects of TMZ. The limitation of the present study was the paucity of the number of enrolled patients. Further studies involving more patients are warranted to confirm the findings of this study.
Conclusions
This is the first clinical trial of combinational therapy with VEGFRs peptide-based vaccines plus TMZ in patients with primary glioblastomas. Paired pre- and post-vaccination specimens suggested that VEGFRs peptide vaccination may possibly enhance the effects of TMZ. Further studies involving more patients are warranted to confirm the findings of this study.
Supplementary information
Additional file 1: Figure S1. Other factors in the tumor microenvironment. The expression of PD-1 and PD-L1 (A), nestin (B), and CD 163, CD8, CD4 (C) in the tumors of pre- and post- vaccination. The number of positive cells did not change after vaccination. (original magnification, × 40; magnification bar, 100 μm; black arrows, positive cells).
Additional file 2: Table S1. Review of reported clinical trials using VEGFR1 or 2 peptide vaccine.
Acknowledgments
The authors greatly thank Ms. Naoko Tsuzaki in the department of Neurosurgery for technical assistance of laboratory works, and the enago group (www.enago.jp/) for editing a draft of this manuscript.
Abbreviations
- BCNU
1,3-bis-Chloroethyl-1-Nitrosourea
- CAN
Chromosomal number aberrations
- CR
Complete response
- CT
Computed tomography
- CTL
Cytotoxic T lymphocytes
- DC
Dendritic cell
- ELISPOT
Enzyme-linked immunoSpot
- FLAIR
Fluid attenuated IR
- Gd
Gadolinium
- GMP
Good manufacturing practice
- HPLC
High-performance liquid chromatography
- IDH
Isocitrate dehydrogenase
- MDSC
Myeloid-derived suppressor cells
- MGMT
O6-methylguanine DNA methyltransferase
- MRI
Magnetic resonance imaging
- MVD
Microvessel density
- OS
Overall survival
- PDGFR
Platelet-derived growth factor receptor
- qPCR
Quantitative real-time PCR
- RT
Radiotherapy
- TAM
Tumor-associated macrophage
- TMZ
Temozolomide
- VEGF
Vascular endothelial growth factor
- VEGFR
Vascular endothelial growth factor receptor
Authors’ contributions
MT is the principal investigator of this study. RT, YM, KK, YO, MS, RK, and HN were study investigators. RT, YM and MT did the statistical analysis. RT, YM, RU, YK, HS, KY and MT designed the clinical trial. RT, YM, KK, YO, MS, RK, HN and SN collected the data. RT, YM, KK, YO, MS, SN, HS, YK, KY, and MT analyzed and interpreted data. All authors contributed to the writing of the report and approved the final version.
Funding
This work was supported in part by grants from the Japan Society for the Promotion of Science (JSPS) (17H04306 and 18K19622 to M.T., and 18 J21382 to R.T.). The role of the funder was data collection and analysis.
Availability of data and materials
All data supporting the findings of this study are available within the article and its Supplementary Information Files and from the corresponding author on reasonable request.
Ethics approval and consent to participate
Written informed consent was obtained from all individual participants included in the study. The authors affirm that all individual participants provided informed consent for publication of their data. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Keio University School of Medicine Ethics Committee (reference number: 20130461) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
All clinical details of participants were anonymized.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12885-020-6589-x.
References
- 1.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhry IH, O’Donovan DG, Brenchley PE, Reid H, Roberts IS. Vascular endothelial growth factor expression correlates with tumour grade and vascularity in gliomas. Histopathology. 2001;39:409–415. doi: 10.1046/j.1365-2559.2001.01230.x. [DOI] [PubMed] [Google Scholar]
- 3.Tamura R, Ohara K, Sasaki H, Morimoto Y, Yoshida K, Toda M. Histopathological vascular investigation of the peritumoral brain zone of glioblastomas. J Neuro-Oncol. 2018;136:233–241. doi: 10.1007/s11060-017-2648-9. [DOI] [PubMed] [Google Scholar]
- 4.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–882. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roskoski R. Vascular endothelial growth factor (VEGF) signaling in tumor progression. Crit Rev Oncol Hematol. 2007;62:179–213. doi: 10.1016/j.critrevonc.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Hamerlik P, Lathia JD, Rasmussen R, Wu Q, Bartkova J, Lee M, Moudry P, Bartek J, Jr, Fischer W, Lukas J, Rich JN, Bartek J. Autocrine VEGF-VEGFR2-Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J Exp Med. 2012;209:507–520. doi: 10.1084/jem.20111424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamura R, Tanaka T, Akasaki Y, Murayama Y, Yoshida K, Sasaki H. The role of vascular endothelial growth factor in the hypoxic and immunosuppressive tumor microenvironment: perspectives for therapeutic implications. Med Oncol. 2019;37:2. doi: 10.1007/s12032-019-1329-2. [DOI] [PubMed] [Google Scholar]
- 8.Batchelor TT, Duda DG, di Tomaso E, Ancukiewicz M, Plotkin SR, Gerstner E, Eichler AF, Drappatz J, Hochberg FH, Benner T, Louis DN, Cohen KS, Chea H, Exarhopoulos A, Loeffler JS, Moses MA, Ivy P, Sorensen AG, Wen PY, Jain RK. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28:2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ, Jr, Mehta MP. A randomized trial of Bevacizumab for newly diagnosed Glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hainsworth JD, Ervin T, Friedman E, Priego V, Murphy PB, Clark BL, Lamar RE. Concurrent radiotherapy and temozolomide followed by temozolomide and sorafenib in the first-line treatment of patients with glioblastoma multiforme. Cancer. 2010;116:3663–3669. doi: 10.1002/cncr.25275. [DOI] [PubMed] [Google Scholar]
- 12.Koutras AK, Krikelis D, Alexandrou N, Starakis I, Kalofonos HP. Brain metastasis in renal cell cancer responding to sunitinib. Anticancer Res. 2007;27:4255–4257. [PubMed] [Google Scholar]
- 13.Ma J, Waxman DJ. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol Cancer Ther. 2008;7:3670–3684. doi: 10.1158/1535-7163.MCT-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 15.Zustovich F, Landi L, Lombardi G, Porta C, Galli L, Fontana A, Amoroso D, Galli C, Andreuccetti M, Falcone A, Zagonel V. Sorafenib plus daily low-dose temozolomide for relapsed glioblastoma: a phase II study. Anticancer Res. 2013;33:3487–3494. [PubMed] [Google Scholar]
- 16.Shibao S, Ueda R, Saito K, Kikuchi R, Nagashima H, Kojima A, Kagami H, Pareira ES, Sasaki H, Noji S, Kawakami Y, Yoshida K, Toda M. A pilot study of peptide vaccines for VEGF receptor 1 and 2 in patients with recurrent/progressive high grade glioma. Oncotarget. 2018;9:21569–21579. doi: 10.18632/oncotarget.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kikuchi R, Ueda R, Saito K, Shibao S, Nagashima H, Tamura R, Morimoto Y, Sasaki H, Noji S, Kawakami Y, Yoshida K, Toda M. A pilot study of vaccine therapy with multiple Glioma Oncoantigen/Glioma angiogenesis-associated antigen peptides for patients with recurrent/progressive high-grade Glioma. J Clin Med. 2019;8:E263. doi: 10.3390/jcm8020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura R, Fujioka M, Morimoto Y, Ohara K, Kosugi K, Oishi Y, Sato M, Ueda R, Fujiwara H, Noji S, Oishi N, Ogawa K, Kawakami Y, Ohira T, Yoshida K, Toda M. A VEGF receptor vaccine demonstrates preliminary efficacy in neurofibromatosis type 2. Nat Commun. 2019;10:5758. doi: 10.1038/s41467-019-13640-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramakrishnan R, Gabrilovich DI. Mechanism of synergistic effect of chemotherapy and immunotherapy of cancer. Cancer Immunol Immunother. 2011;60:419–423. doi: 10.1007/s00262-010-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyazawa M, Ohsawa R, Tsunoda T, Hirono S, Kawai M, Tani M, Nakamura Y, Yamaue H. Phase I clinical trial using peptide vaccine for human vascular endothelial growth factor receptor 2 in combination with gemcitabine for patients with advanced pancreatic cancer. Cancer Sci. 2010;101:433–439. doi: 10.1111/j.1349-7006.2009.01416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, Altiok S, Celis E, Gabrilovich DI. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120:1111–1124. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, Ellingson BM, Hashimoto N, Pollack IF, Brandes AA, Franceschi E, Herold-Mende C, Nayak L, Panigrahy A, Pope WB, Prins R, Sampson JH, Wen PY, Reardon DA. Immunotherapy response assessment in neurooncology: a report of the RANO working group. Lancet Oncol. 2015;16:e534–e542. doi: 10.1016/S1470-2045(15)00088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 24.Ranieri E, Popescu I, Gigante M. CTL ELISPOT assay. Methods Mol Biol. 2014;1186:75–86. doi: 10.1007/978-1-4939-1158-5_6. [DOI] [PubMed] [Google Scholar]
- 25.Hirose Y, Aldape K, Takahashi M, Berger MS, Feuerstein BG. Tissue microdissection and degenerate oligonucleotide primed-polymerase chain reaction (DOP-PCR) is an effective method to analyze genetic aberrations in invasive tumors. J Mol Diagn. 2001;3:62–67. doi: 10.1016/S1525-1578(10)60653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamura R, Tanaka T, Ohara K, Miyake K, Morimoto Y, Yamamoto Y, Kanai R, Akasaki Y, Murayama Y, Tamiya T, Yoshida K, Sasaki H. Persistent restoration to the immunosupportive tumor microenvironment in glioblastoma by bevacizumab. Cancer Sci. 2018;110:499–508. doi: 10.1111/cas.13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura R, Tanaka T, Miyake K, Tabei Y, Ohara K, Sampetrean O, Kono M, Mizutani K, Yamamoto Y, Murayama Y, Tamiya T, Yoshida K, Sasaki H. Histopathological investigation of glioblastomas resected under bevacizumab treatment. Oncotarget. 2016;7:52423–52435. doi: 10.18632/oncotarget.9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamura R, Ohara K, Morimoto Y, Kosugi K, Oishi Y, Sato M, Yoshida K, Toda M. PITX2 expression in non-functional pituitary neuroendocrine tumor with cavernous sinus invasion. Endocr Pathol. 2019;30:81–89. doi: 10.1007/s12022-019-9573-8. [DOI] [PubMed] [Google Scholar]
- 29.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 30.Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, Carbone DP. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878–4886. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 31.Li YL, Zhao H, Ren XB. Relationship of VEGF/VEGFR with immune and cancer cells: staggering or forward? Cancer Biol Med. 2016;13:206–214. doi: 10.20892/j.issn.2095-3941.2015.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. 2018;9:978. doi: 10.3389/fimmu.2018.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto Y, Tamura R, Tanaka T, Ohara K, Tokuda Y, Miyake K, Takei J, Akasaki Y, Yoshida K, Murayama Y, Sasaki H. "paradoxical" findings of tumor vascularity and oxygenation in recurrent glioblastomas refractory to bevacizumab. Oncotarget. 2017;8:103890–103899. doi: 10.18632/oncotarget.21978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamura R, Tanaka T, Miyake K, Yoshida K, Sasaki H. Bevacizumab for malignant gliomas: current indications, mechanisms of action and resistance, and markers of response. Brain Tumor Pathol. 2017;34:62–77. doi: 10.1007/s10014-017-0284-x. [DOI] [PubMed] [Google Scholar]
- 35.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akasaki Y, Kikuchi T, Homma S, Koido S, Ohkusa T, Tasaki T, Hayashi K, Komita H, Watanabe N, Suzuki Y, Yamamoto Y, Mori R, Arai T, Tanaka T, Joki T, Yanagisawa T, Murayama Y. Phase I/II trial of combination of temozolomide chemotherapy and immunotherapy with fusions of dendritic and glioma cells in patients with glioblastoma. Cancer Immunol Immunother. 2016;65:1499–1509. doi: 10.1007/s00262-016-1905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batich KA, Reap EA, Archer GE, Sanchez-Perez L, Nair SK, Schmittling RJ, Norberg P, Xie W, Herndon JE, 2nd, Healy P, McLendon RE, Friedman AH, Friedman HS, Bigner D, Vlahovic G, Mitchell DA, Sampson JH. Long-term Survival in Glioblastoma with Cytomegalovirus pp65-Targeted Vaccination. Clin Cancer Res. 2017;23:1898–1909. doi: 10.1158/1078-0432.CCR-16-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki H, Onishi H, Wada J, Yamasaki A, Tanaka H, Nakano K, Morisaki T, Katano M. VEGFR2 is selectively expressed by FOXP3high CD4+ Treg. Eur J Immunol. 2010;40:197–203. doi: 10.1002/eji.200939887. [DOI] [PubMed] [Google Scholar]
- 40.Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, Dubreuil O, Carpentier AF, Tartour E, Taieb J. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73:539–549. doi: 10.1158/0008-5472.CAN-12-2325. [DOI] [PubMed] [Google Scholar]
- 41.Hazama S, Nakamura Y, Takenouchi H, Suzuki N, Tsunedomi R, Inoue Y, Tokuhisa Y, Iizuka N, Yoshino S, Takeda K, Shinozaki H, Kamiya A, Furukawa H, Oka M. A phase I study of combination vaccine treatment of five therapeutic epitope-peptides for metastatic colorectal cancer; safety, immunological response, and clinical outcome. J Transl Med. 2014;12:63. doi: 10.1186/1479-5876-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hazama S, Nakamura Y, Tanaka H, Hirakawa K, Tahara K, Shimizu R, Ozasa H, Etoh R, Sugiura F, Okuno K, Furuya T, Nishimura T, Sakata K, Yoshimitsu K, Takenouchi H, Tsunedomi R, Inoue Y, Kanekiyo S, Shindo Y, Suzuki N, Yoshino S, Shinozaki H, Kamiya A, Furukawa H, Yamanaka T, Fujita T, Kawakami Y, Oka M. A phase ΙI study of five peptides combination with oxaliplatin-based chemotherapy as a first-line therapy for advanced colorectal cancer (FXV study) J Transl Med. 2014;12:108. doi: 10.1186/1479-5876-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iinuma H, Fukushima R, Inaba T, Tamura J, Inoue T, Ogawa E, Horikawa M, Ikeda Y, Matsutani N, Takeda K, Yoshida K, Tsunoda T, Ikeda T, Nakamura Y, Okinaga K. Phase I clinical study of multiple epitope peptide vaccine combined with chemoradiation therapy in esophageal cancer patients. J Transl Med. 2014;12:84. doi: 10.1186/1479-5876-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishizaki H, Tsunoda T, Wada S, Yamauchi M, Shibuya M, Tahara H. Inhibition of tumor growth with antiangiogenic cancer vaccine using epitope peptides derived from human vascular endothelial growth factor receptor 1. Clin Cancer Res. 2006;12:5841–5849. doi: 10.1158/1078-0432.CCR-06-0750. [DOI] [PubMed] [Google Scholar]
- 45.Masuzawa T, Fujiwara Y, Okada K, Nakamura A, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Osawa R, Takeda K, Yoshida K, Tsunoda T, Nakamura Y, Mori M, Doki Y. Phase I/II study of S-1 plus cisplatin combined with peptide vaccines for human vascular endothelial growth factor receptor 1 and 2 in patients with advanced gastric cancer. Int J Oncol. 2012;41:1297–1304. doi: 10.3892/ijo.2012.1573. [DOI] [PubMed] [Google Scholar]
- 46.Wada S, Tsunoda T, Baba T, Primus FJ, Kuwano H, Shibuya M, Tahara H. Rationale for antiangiogenic cancer therapy with vaccination using epitope peptides derived from human vascular endothelial growth factor receptor 2. Cancer Res. 2005;65:4939–4946. doi: 10.1158/0008-5472.CAN-04-3759. [DOI] [PubMed] [Google Scholar]
- 47.Yoshimura K, Minami T, Nozawa M, Uemura H. Phase I clinical trial of human vascular endothelial growth factor receptor 1 peptide vaccines for patients with metastatic renal cell carcinoma. Br J Cancer. 2013;108:1260–1266. doi: 10.1038/bjc.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu Y, Cui J. Present and future of cancer immunotherapy: a tumor microenvironmental perspective. Oncol Lett. 2018;16:4105–4113. doi: 10.3892/ol.2018.9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Other factors in the tumor microenvironment. The expression of PD-1 and PD-L1 (A), nestin (B), and CD 163, CD8, CD4 (C) in the tumors of pre- and post- vaccination. The number of positive cells did not change after vaccination. (original magnification, × 40; magnification bar, 100 μm; black arrows, positive cells).
Additional file 2: Table S1. Review of reported clinical trials using VEGFR1 or 2 peptide vaccine.
Data Availability Statement
All data supporting the findings of this study are available within the article and its Supplementary Information Files and from the corresponding author on reasonable request.