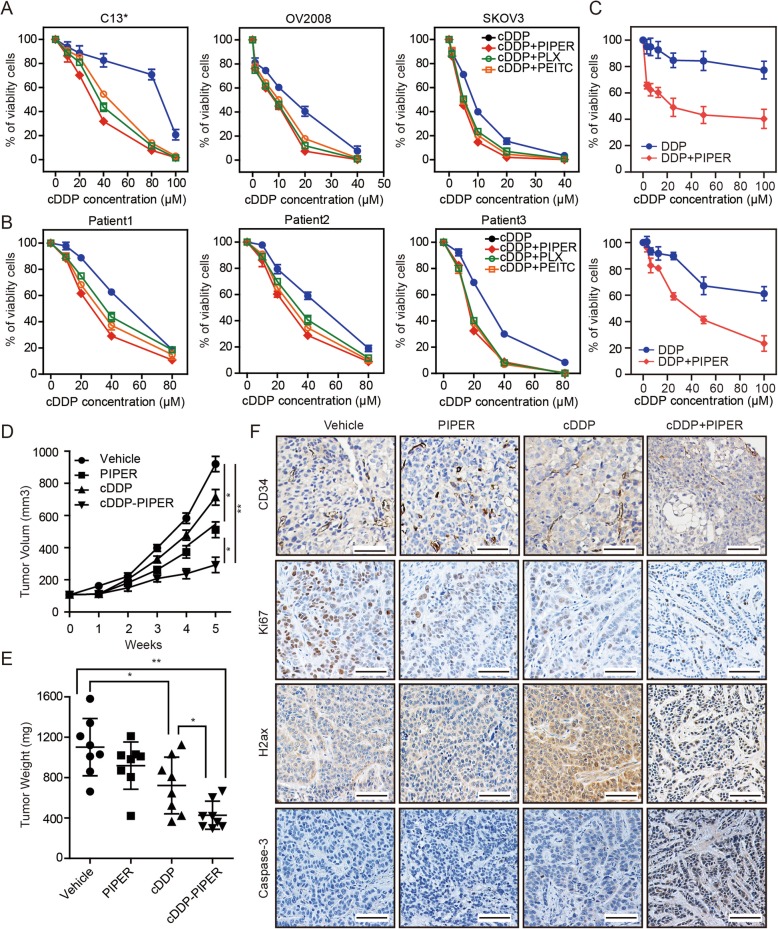
Fig. 1.
ROS levels are associated with cDDP sensitivity of ovarian cancer. a cDDP IC50 curves for ovarian cancer cell lines C13*, OV2008 and SKOV3 with or without ROS-elevating drugs (PLX4032, 1 μM, Piperlongumine (PIPER, 10 μM) and β-phenylethyl isothiocyanate (PEITC, 10 μM)). b Cell viability of 3 strains of primary cancer cells was assayed after treatment with increasing concentrations of cDDP with or without ROS-elevating drugs for 48 h by CCK-8. c Cell viability of primary cancer cells derived from patients with recurrent ovarian cancer or primary ovarian cancer was assayed after treatment with increasing concentrations of cDDP with or without PIPER for 48 h by CCK-8. a-c The two-tailed P-values < 0.05 were considered to indicate statistically significant differences. The results were tested by three independent experiments. d Growth curves of C13* subcutaneous xenograft tumors treated with vehicle, cDDP (2 mg/kg, intraperitoneally every 4 days), PIPER (2 mg/kg, intraperitoneally daily for 28 consecutive days), and cDDP plus PIPER (same dose as used in the single-agent groups) are shown. Tumor volumes were calculated as length × (square of width)/2. n = 8 per group. (*P < .05, **P < .001, two-sided Student t-test). e Tumor weights in nude mice were measured on day 35 after tumor cell injection. n = 8 per group. (*P < .05, **P < .001, two-sided Student t-test). f The immunohistochemistry analyses for caspase 3, Ki67, γ-H2AXand CD34 staining were carried out on C13* xenograft tumor sections collected from mice treated with the indicated treatments. Representative staining is shown. Scale bars = 50 μm. Data in (a–e) are the mean values ±95% confidence intervals