Figure 1: Regulation of Triglyceride-Enriched Lipoprotein Lipolysis.
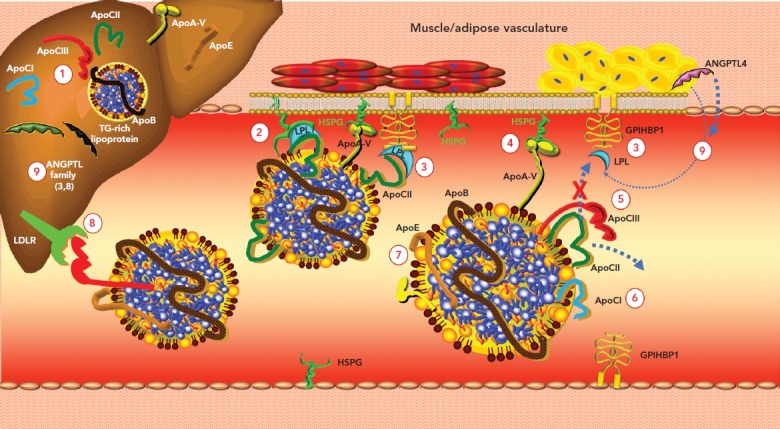
1. Apolipoprotein (apo) CIII stimulates hepatic triglyceride (TG)-rich lipoprotein production. 2. ApoCII is an essential cofactor and activator of lipoprotein lipase (LPL), which is anchored to heparan sulphate proteoglycan (HSPG) and glycosylphosphatidylinositol-anchored HDL-binding protein 1 (GPIHBP1). These receptors are crucial for maintaining LPL activity. 3. GPIHBP1 seizes LPL in the interstitial space and shuttles it across endothelial cells to the luminal surface; it also tethers LPL to the capillary endothelium. 4. ApoA-V binds to HSPG and activates LPL. 5. ApoCIII inhibits LPL-mediated lipolysis by both displacing the LPL activator apoCII from the lipoprotein surface and blocking the interaction of apoCII with LPL (steric hindrance). 6. ApoCI inhibits LPL activity by preventing binding of LPL to TG-rich lipoproteins and by rendering LPL more susceptible to inhibition by angiopoietin-like protein (ANGPTL) 4. 7. ApoE2 can interfere with LPL. 8. ApoCIII inhibits receptor-mediated hepatic clearance of TG-rich lipoproteins. 9. ANGPTL3, ANGPTL4 and ANGPTL8 are members of a family of secreted proteins that inhibit LPL. Source: Sathiyakumar et al. 2018.[155] Reproduced with permission from Elsevier.
