Table 1.
Half-potential and catalytic reaction yield of iron complexes used to couple pyrrole and phenylboronic acid to produce 2-phenylpyrrole using 10% catalyst loading in the presence of air.
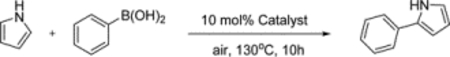 | ||
|---|---|---|
| Catalyst | Yield (%)a | E1/2 (mV)b |
| [Fe3+L1(Cl)2]+ | 57 ± 3 | −465 |
| [Fe3+L4(Cl)2]+ | 60 ± 1 | −285 |
| [Fe2+L5(Cl)]+ | 30 ± 2 | −442 |
| [Fe2+L6(Cl)2] | 81 ± 7 | −405 |
| [Fe3+L7(Cl)2]+ | 74 ± 3 | −391 |
| [Fe3+L8(Cl)2]+ | 68 ± 4 | −306 |
| [Fe2+L9(Cl)]+ | 32 ± 5 | −285 |
| [Fe2+L10(Cl)]+ | 19 ± 2 | 73 |
| L3 + FeC2O4 ·2H2O | 61 ± 5 | - |
Yields determined by NMR analysis.
Referenced vs. Fc/Fc+ 0.0 mV.
