Figure 1.
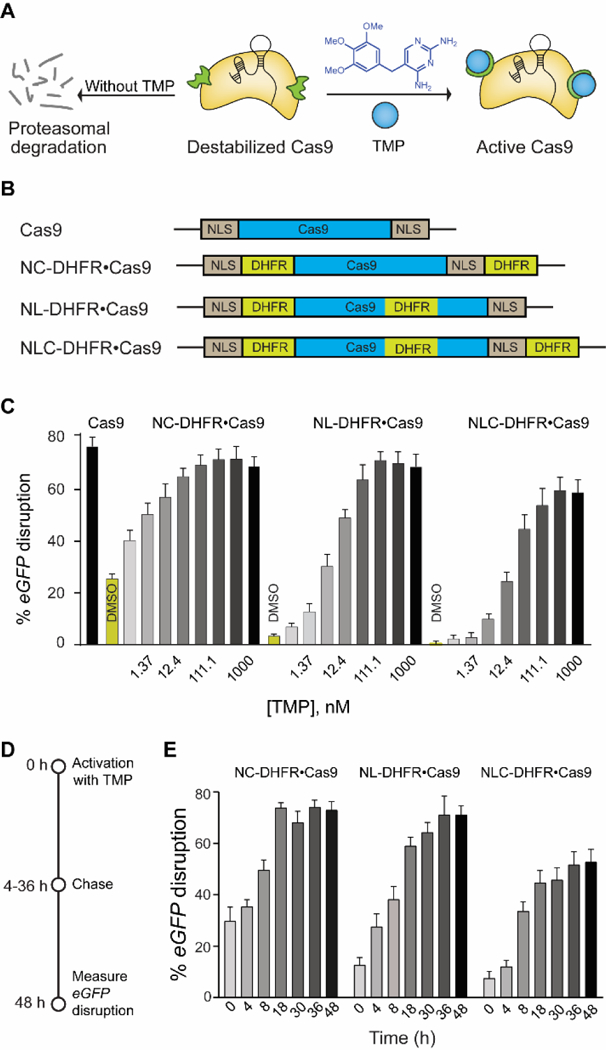
(A) Schematic representation of destabilized DHFR domains fused to Cas9, leading to proteosomal degradation. TMP stabilizes the DHFR•Cas9 fusion protein to generate active Cas9. (B) Schematic representation of different DHFR-fused Cas9 constructs. (C) Dose-dependent activation of different DHFR•Cas9 systems in the eGFP-disruption assay in U2OS cells (D) Schematic representation of reversible activation of Cas9. Cells were nucleofected with different DHFR•Cas9 constructs and treated with TMP (37 nM). The media was then removed and fresh media without TMP was added at different time intervals over 48 h, and the cells were imaged after 48 h. (E) Comparison of reversible and dose dependent activation of three different DHFR•Cas9 constructs by TMP (37 nM).
