Abstract
Purpose:
Evaluate risk factors for cytomegalovirus (CMV) reactivation during the first year after kidney transplantation in the CMV seropositive older recipient.
Methods:
Retrospective single-center study.
Results:
Between 2011-2015, 91 patients ≥65 years received a kidney transplant; these were matched with 91 controls, aged 40-60. Risk of CMV reactivation in the CMV seropositive recipients was analyzed. Sixty-three older and 54 younger recipients were included; 50% had received CMV-directed prophylaxis. CMV reactivation was significantly more frequent in the older group (71.4% vs 44.4%, p=0.003) and occurred earlier (p=0.003). A multivariate model showed that only age was associated with CMV reactivation (OR 2.48, p=0.03). After excluding patients that received thymoglobulin, older age group remained the only risk factor of CMV reactivation (OR 3.81, p=0.014). Recurrent event analysis showed that the older cohort had an HR of 1.94 (p=0.01) of CMV viremia; there was significant episode-cohort interaction (p<0.01). While the older group had a higher risk of infection (HR=2.43), after the initial episode the relative hazards were approximately equal (HR=1.08, at period 2).
Conclusion:
This suggests that it is key to specifically avoid the first episode of reactivation. Universal prophylaxis or a hybrid prophylaxis model should be considered in the CMV seropositive kidney transplant recipient aged ≥65 years.
Keywords: Older Adults, Cytomegalovirus, Kidney Transplantation, Infection
Introduction
Older adults are at higher risk of infectious complications[1,2] due to immunosenescence, frailty, functional impairment and comorbidities. In older kidney transplant (KT) patients, immunosuppressive therapies further increase the risk of infections and chances of a poor outcome[3-6]. Cytomegalovirus (CMV) infection is one of the most frequent and severe infectious complications after kidney transplantation[7]. Older KT recipients have a high prevalence of CMV seropositivity and accordingly are a growing population at risk for this infectious complication [8-10].
CMV seronegative KT recipients that receive an organ from a seropositive donor are at highest risk for CMV viremia and disease and typically receive universal prophylaxis with an anti-CMV active antiviral, usually ganciclovir or valganciclovir[7]. KT recipients at intermediate risk for CMV infection (CMV seropositive recipients of a seronegative or seropositive organ) can receive either universal prophylaxis or undergo pre-emptive therapy consisting of weekly CMV polymerase chain reaction (PCR) monitoring for 3 months and receipt of antiviral therapy only if viremia is documented[7].
Several studies compared pre-emptive therapy versus universal prophylaxis in KT recipients at intermediate risk for CMV[11-22], but there is a gap in knowledge regarding which is the best approach in the growing population of older seropositive solid organ transplant recipients. We previously described an increased risk of CMV infection in older adults compared to younger adults after KT[23]. In fact, older adults were twice as likely to reactivate CMV compared to the younger group (p=0.012). We therefore performed a study to determine whether age is a risk for CMV reactivation in the CMV seropositive (CMV R+) KT population, as this would potentially influence the type of preventive approach employed for older seropositive KT recipients.
Material and methods
Study design and Study Center
This was a single-center retrospective cohort study of kidney-only transplants performed between 2011-2015 at Duke University Medical Center in Durham, NC; 551 KT were performed during the study period. Older KT recipients were defined as adults aged ≥65 years; younger KT recipients or “controls” were 40-60 years of age. The study was approved by the Duke University Health System Institutional Review Board for Clinical Investigation (Pro00076804).
Patient cohorts
An institutional tool, the Duke Enterprise Data Unified Content Explorer (DEDUCE)[24], was used to identify all KT recipients during the 5-year study period and their age. All 91 patients aged ≥65 that received a kidney-only transplant were included. Of the 257 potential controls aged 40-60 years, 91 patients were randomly matched 1:1 to the older KT recipients by year of transplantation, sex and, if possible, race. For this analysis only the CMV R+ subpopulation was included.
Antiviral prophylaxis for CMV recipient positive recipients
Antiviral prophylaxis for CMV R+ patients consisted of pre-emptive monitoring with weekly CMV PCR tests for 3 months and initiation of antiviral therapy (ganciclovir or valganciclovir) in patients with a CMV viral load of 450 IU/ml or above. CMV R+ recipients that received anti-thymocyte globulin induction received universal prophylaxis with ganciclovir/valganciclovir for 180 days followed by CMV PCR monitoring every 2 weeks for a minimum of 3 months.
Data Extraction and CMV definitions
Demographic, clinical, microbiological and outcome data were extracted manually from the medical charts. Data collected were managed using REDCap™ electronic data capture tool hosted at Duke[25]. Infection data collection included information about infectious syndromes and PCR. Standard CMV definitions and definitions per CDC/NSHN as described elsewhere were used[26,27]. At our institution, the threshold of detection of CMV is 137 IU/ml and this was used to define a positive CMV PCR, the primary endpoint of the study. Our secondary endpoint was clinically significant CMV disease defined as CMV syndrome, tissue-invasive disease or a CMV PCR of 450 IU/ml, all of which resulted in initiation of antiviral therapy. A comprehensive list of previously identified risk factors for CMV reactivation[28,29]or factors thought to be of clinical importance in the authors’ views was used for the risk analysis.
Statistical Analysis
Descriptive results are shown as total numbers/percentages, mean/standard deviations (SD) and medians/interquartile range (IQR). Differences between groups were analyzed using the chi-square goodness-of-fit or t-test. The analysis of CMV infection (including any CMV viremia over the above mentioned threshold) was parameterized in several ways, each assessing different aspects of the disease process; presence-absence over specific time periods, time to first CMV infection, number of episodes of CMV viremia; each estimated by different models: Chi-square (for tests of proportions), time to event (for assessment of time to CMV infection post-transplant), Poisson regression (to assess group differences in number of infections). Several analyses were performed. First, the difference by age group of any versus no CMV infection; second, the difference in the total number of episodes within a period. Third, a multivariable prediction model for CMV was developed, combining the two age cohorts to assess which set of variables best predict CMV during the first year. A sub-analysis was performed excluding patients that received thymoglobulin as they were considered at high risk for CMV reactivation and had received universal CMV prophylaxis followed by preemptive monitoring as outlined above. Time to first CMV viremia post-transplant was tested by Kaplan-Meier techniques and the log-rank test. Patients were censored at death, or at the one-year mark after transplant, whichever occurred first. Marginal modeling for recurrent events was used to analyze recurrent episodes of CMV reactivation. Statistical analysis was performed using R studio (Version 1.1.463) for R.
Results
Baseline characteristics
The total cohort included 117 CMV R+ kidney transplant recipients, including 63 (53.85%) older and 54 (46.15%) younger adults. Baseline characteristics are shown in Table 1.
Table 1. Baseline characteristics of CMV recipient positive transplant recipients.
ATG: anti-thymocyte globulin; CMV: cytomegalovirus; CVD: cardiovascular disease; IQR: interquartile range; KT: kidney transplant; MMF: mycophenolate mofetil; PRA: panel reactive antibody.
| Variable | Total n (%) n=117 |
|---|---|
| Age in years, median [IQR] | 65 (50,68) |
| Older cohort | 63 (53.85) |
| Sex female | 50 (42.74) |
| Race | |
| African-American | 43 (36.75) |
| Caucasian | 67 (57.26) |
| Asian | 7 (5.98) |
| Comorbidities | |
| Diabetes mellitus | 42 (35.90) |
| Hypertension | 105 (89.74) |
| CVD | 42 (35.90) |
| Prior transplant | 21 (17.95) |
| Prior KT | 16 (76.19) |
| PRA in percentage, median [IQR] | 0 [0,52] |
| Induction regimen | |
| Basiliximab | 61 (52.14) |
| ATG | 40 (34.19) |
| Steroids only | 18 (15.38) |
| Ureteral stent used | 91 (77.78) |
| Maintenance immunosuppression | |
| Prednisone | 115 (98.29) |
| MMF | 114 (97.44) |
| Tacrolimus | 116 (99.15) |
| Length of hospital stay in days, median [IQR] | 6 [5,10] |
| Delayed graft function | 28 (23.93) |
| Discharged home | 117 (100%) |
| CMV seropositive donor | 86 (73.50) |
| CMV directed therapy (at least 30 days) | 59 (50.43) |
| Death | 5 (4.27) |
| Graft loss | 3 (2.56) |
| Functional status before transplant (available in n=115) | |
| Independent for ambulation | 99 (86.09) |
| Dependent for ambulation (cane, walker or need help) | 16 (13.91) |
| Functional status after transplant (available in n=98) | |
| Independent for ambulation | 76 (77.55) |
| Dependent for ambulation (cane, walker or need help) | 22 (22.45) |
CMV viremia – Primary endpoint
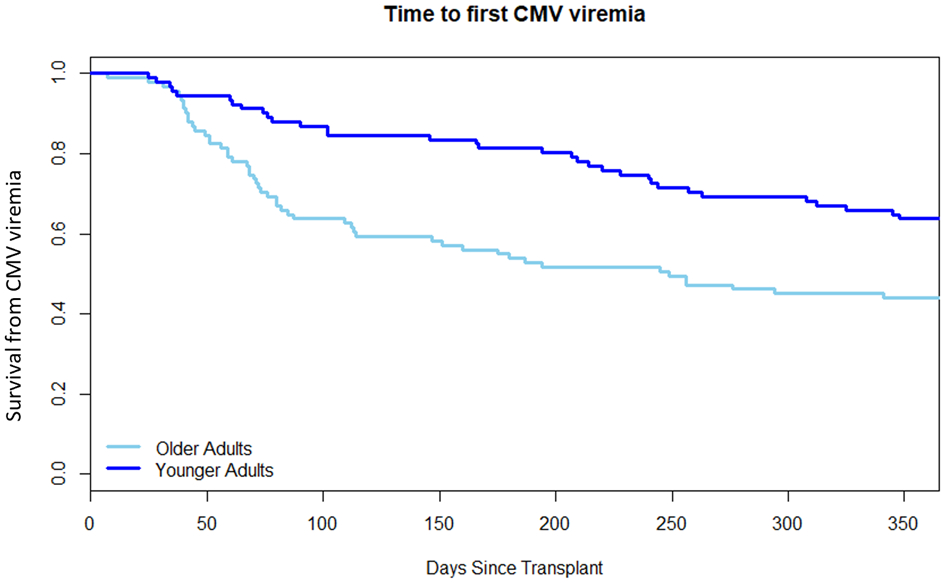
Forty-five older adults (71.43%) had at least one positive CMV PCR test compared to 24 (44.44%) of the younger adults (p=0.003). The odds of an older adult having a positive CMV test was 3.13 times higher (95% CI 1.45, 6.72) than the odds of someone of the younger cohort. The total number of episodes of any CMV viremia was higher in the older compared to the younger cohort, although not statistically significant; 69 versus 49 episodes (p=0.07). See Figure 1. Cox regression analysis showed a hazard ratio (HR) of 2.30 (95% CI 1.40, 3.80) of an episode of CMV in the older CMV R+ age group (p=0.001). Time to first viremia by age group is shown in Figure 2 (p=0.003), median time to first positivity was 68 days in the older group (IQR 7, 109) and 96 days in the younger group (IQR 25, 215.5).
Fig. 1.
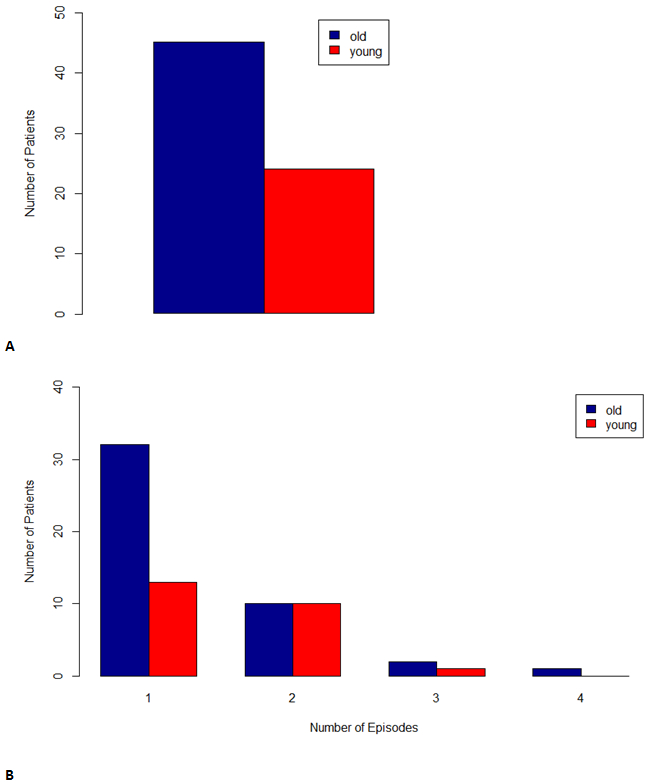
A. Number of patients with any CMV viremia in the older versus younger cohort. B. Number of episodes of CMV viremia in the first year after kidney transplantation in the older versus younger cohort.
Fig. 2. Time to first episode of CMV viremia. p=0.003.
A univariate analysis of variables suspected to be risk factors for CMV reactivation for the whole cohort is shown in Table 2. All variables with a p ≤ 0.1 were carried forward into the multivariate model. Results of the multivariate model are shown in Table 3. Age group was the only variable significantly associated with CMV reactivation, with the older cohort having an odds of 2.48 (95% CI 1.10, 5.72) of CMV reactivation (p=0.03).
Table 2. Univariate logistic regression analysis of potential risk factor in predicting CMV reactivation in CMV seropositive kidney transplant recipients.
| Univariate Logistic Regression (n = 117) | |||
|---|---|---|---|
| Variable | n (%) | p | Odds ratio (95% CI) |
| Older age cohort | 63 (53.85) | 0.003 | 1.31 (1.10,1.56) |
| Sex female | 50 (42.74) | 0.855 | 0.98 (0.82,1.18) |
| DM | 42 (35.90) | 0.0144 | 1.26 (1.05, 1.51) |
| Prior transplant | 21 (17.95) | 0.0319 | 0.78 (0.82,0.98) |
| CMV seropositive donor | 86 (73.50) | 0.589 | 1.06 (0.86,1.30) |
| No induction (=steroids only) | 18 (15.38) | 0.404 | 0.90 (0.70,1.15) |
| Basiliximab induction | 61 (52.14) | 0.023 | 1.23 (1.03,1.47) |
| Rejection | 15 (12.8) | 0.638 | 0.94 (0.72, 1.23) |
| Augmented I/S | 14 (11.97) | 0.471 | 0.90 (0.69, 1.20) |
| BKV | 31 (27.19) | 0.724 | 0.96 (0.79, 1.18) |
| Use of CMV directed ppx for at least 30 days (GCV/VGCV) | 59 (50.43) | 0.411 | 1.08(0.90,1.29) |
| Bacteremia | 17 (14.30) | 0.99 | 1.00 (0.77,1.29) |
| Pneumonia | 13 (11.11) | 0.43 | 1.12 (0.84,1.49) |
| Urinary tract infection | 51 (43.59) | 0.729 | 1.03 (0.86, 1.24) |
| Admission for infection [patient level] ¥ | 52 (44.44) | 0.382 | 1.08 (0.91, 1.30) |
| Surgery [patient level] ¥¥ | 30 (25.64) | 0.577 | 1.06 (0.86, 1.30) |
| Ambulation before transplant (independent/needs assistance/dependent) ¥¥¥ | 99 (84.62) | 0.365 | 0.92 (0.78, 1.10) |
52 patients with at least one admission for infection.
30 patients with at least 1 re-operation/surgery (39 total episodes).
Missing in 2 cases. DM: diabetes mellitus; I/S: immunosuppression; BKV: BK polyomavirus; ppx: prophylaxis; GCV: ganciclovir; VGCV: valganciclovir.
Table 3. Multivariate logistic regression analysis in predicting the risk of CMV reactivation in CMV serospositive kidney transplant recipients.
| Multivariate Logistic Regression | |||
|---|---|---|---|
| Variable | ß coefficient | p | Odds ratio (95% CI) |
| Older cohort | 0.91 | 0.03 | 2.48 (1.10, 5.72) |
| Diabetes mellitus | 0.54 | 0.24 | 1.71 (0.70, 4.3) |
| Prior transplant | −0.69 | 0.19 | 0.50 (0.17, 1.40) |
| Basiliximab induction | 0.74 | 0.07 | 2.11 (0.95, 4.75) |
Clinically significant CMV disease – Secondary Endpoints
There was only one episode of CMV syndrome in the older group, no tissue-invasive disease was documented. Additionally, no significant difference between groups was observed in number of patients with a CMV PCR above the pre-established threshold of 450 IU/ml: 30 older (47.6%) and 22 younger adults (40.7), p=0.459.
Non-anti-thymoglobulin cohorts
A total of 77 patients (65.8%) remained after removing KT recipients that had received thymoglobulin during the first year. Table 4 shows the result of the univariate analysis of risk factors for CMV reactivation. The multivariate analysis was only done for the group with any positive CMV PCR (i.e. ≥137 IU/ml), that is 77 patients. Older age was associated with having a positive CMV PCR with an OR of 3.81 (95% CI 1.33, 11.43), p=0.0140, ß coefficient 1.34.
Table 4. Univariate logistic regression analysis of potential risk factor in predicting CMV reactivation in CMV seropositive kidney transplant recipients that did not receive thymoglobulin.
DM: diabetes mellitus; I/S: immunosuppression; BKV: BK polyomavirus; ppx: prophylaxis; GCV: ganciclovir; VGCV: valganciclovir.
| Univariate Logistic Regression (n=77) | |||||
|---|---|---|---|---|---|
| Variable | n (%) | Any CMV PCR | CMV PCR 450+ IU/ml¥ | ||
| p | Odds ratio (95% CI) | p | Odds ratio (95% CI) | ||
| Older age cohort | 45 (58.44) | 0.0008 | 1.44 (1.17,1.76) | 0.174 | 1.17 (0.93, 1.47) |
| Sex female | 26 (33.77) | 0.954 | 1.01 (0.80, 1.27) | 0.688 | 1.05 (0.83, 1.33) |
| DM | 32 (42.86) | 0.0413 | 1.25 (1.01, 1.55) | 0.163 | 1.18 (0.94,1.48) |
| Prior transplant | 8 (10.39) | 0.012 | 0.64 (0.46,0.90) | 0.0407 | 0.68 (0.48, 0.98) |
| CMV seropositive donor | 49 (66.64) | 0.117 | 1.20 (0.96, 1.49) | 0.327 | 1.12 (0.89, 1.42) |
| No induction (=steroids only) | 18 (23.38) | 0.133 | 0.822 (0.64, 1.06) | 0.452 | 0.90 (0.69, 1.18) |
| Basiliximab induction | 59 (76.62) | 0.133 | 1.22 (0.95, 1.56) | 0.452 | 1.11 (0.85, 1.45) |
| Rejection $$ | 9 (11.69) | 0.537 | 0.90 (0.64, 1.26) | 0.884 | 0.97 (0.69, 1.38) |
| Augmented I/S | 9 (11.69) | 0.356 | 0.85 (0.60, 1.20) | 0.585 | 0.90 (0.62, 1.30) |
| BKV | 20 (25.97) | 0.279 | 1.15 (0.90, 1.46) | 0.172 | 1.20 (0.93, 1.54) |
| Use of CMV directed ppx for at least 30 days (GCV/VGCV) | 13 (15.58) | 0.782 | 0.96 (0.72, 1.28) | 0.963 | 0.99 (0.73, 1.34) |
| Bacteremia | 15 (19.48) | 0.66 | 0.94 (0.72, 1.23) | 0.565 | 0.92 (0.69, 1.22) |
| Pneumonia | 12 (15.58) | 0.893 | 1.02 (0.76, 1.37) | 0.388 | 1.15 (0.84, 1.56) |
| Admission for infection [patient level] | 36 (46.75) | 0.769 | 1.03 (0.83, 1.28) | 0.327 | 1.12 (0.89, 1.40) |
| Surgery [patient level] | 20 (25.97) | 0.597 | 0.94 (0.73, 1.20) | 0.739 | 1.04 (0.81, 1.35) |
n=52
Nine patients with rejection, 2 of them had 2 episodes.
Recurrent-event analysis
Marginal models showed that older adults had a HR of CMV viremia of 1.94 (95% CI 1.15, 2.78) when compared to the younger cohort (p=0.0131). There was significant episode-cohort interaction (p<0.001), see Figure 3. The two groups differed in the risk of the 1st episode (HR=2.45). However, for subsequent episodes, the HR was near unity (HR=1.14 for the second episode), indicating no difference in risk. When removing the patients that received thymoglobulin the significant episode-cohort interaction persisted (p<0.001).
Fig. 3. Marginal models. Episode-cohort interaction. First three episodes.
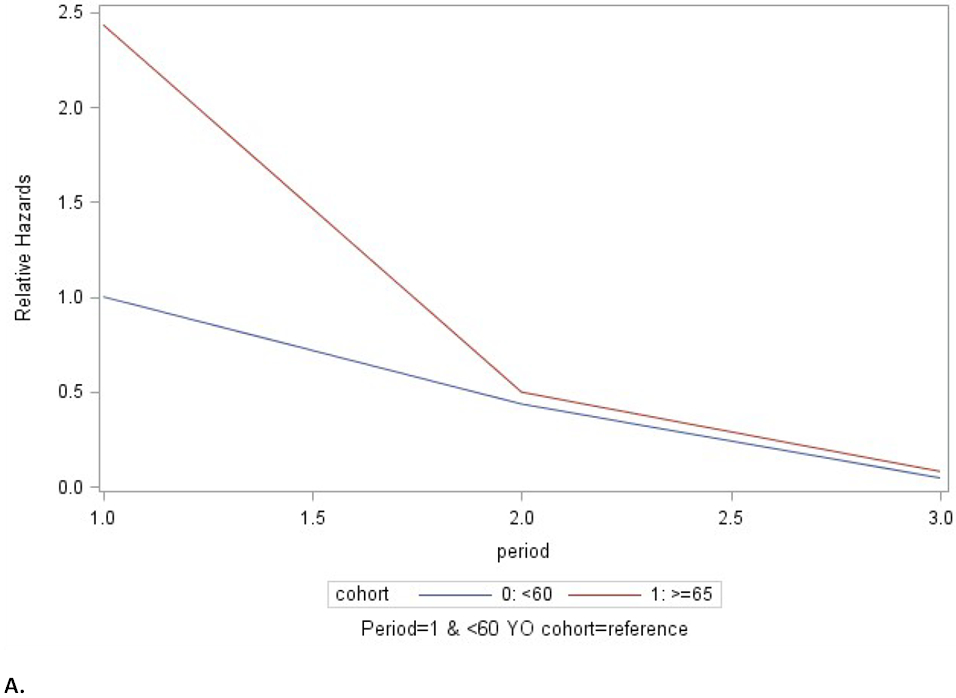
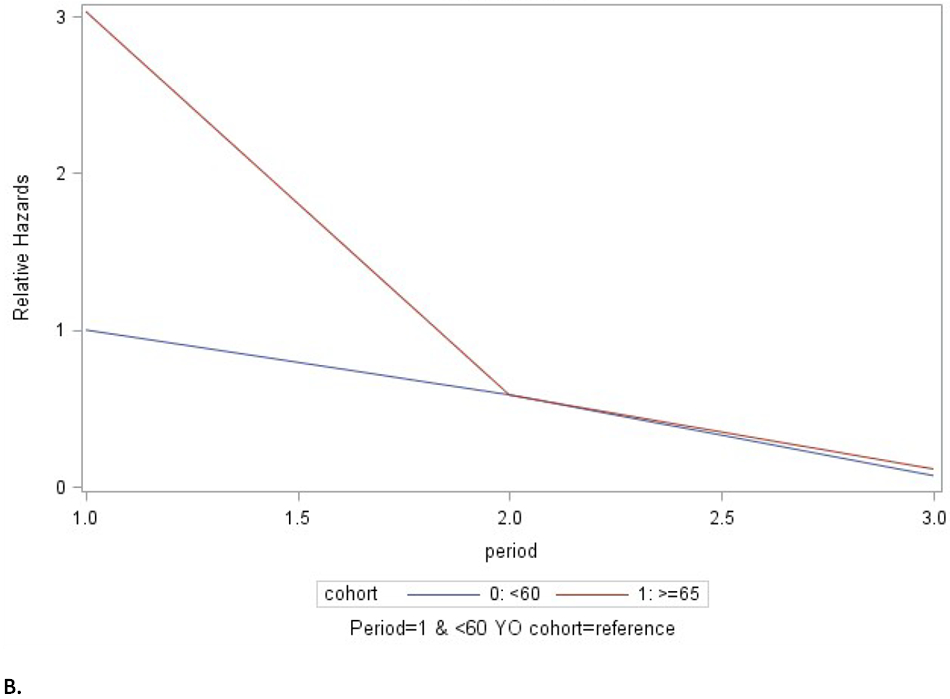
A. All patients. B. Excludes patients that received thymoglobulin in the first year after kidney transplantation. Cohort 0 (blue): 40-60 years of age. Cohort 1 (red): aged ≥ 65 years. Hz: hazard ratio. While the older group had a higher risk of CMV reactivation, after the initial episode the relative hazards of were approximately equal between the age groups (at period 2).
Discussion
Our results confirm that age is an important risk factor for CMV reactivation in the older (age 65 years or older) KT population. In fact, the odds of the older KT population having a positive CMV PCR in the first year after transplantation was 3.13 times (95% CI 1.45, 6.72) more likely than their younger peers (aged 40-60). Further, one episode of CMV reactivation led to a higher risk of subsequent episodes of CMV reactivation and treatment.
Others studies have also found age to be associated with CMV reactivation risk in CMV seropositive patients albeit with a HR of 1.02 (95% CI 1.01, 1.04) and in a younger (mean age 53.4 years) cohort [20]. D+/R+ KT recipients aged 55 years and older undergoing pre-emptive monitoring and with CMV reactivation had poorer 5-year survival than those without CMV reactivation[16]. Additionally, and mirroring our experience, most infections took place in the first 6 months after transplantation[16,23].
CMV is thought to be one of the drivers of the pro-inflammatory process that goes along with aging, also known as “inflammaging” [30]. CMV has been associated with frailty and mortality[31,32] by a series of mechanisms that involve T-cell expansion, increase in the production of cytokines and the state of chronic inflammation. These factors are associated with other comorbidities e.g. cardiovascular disease, diabetes mellitus, autoimmune diseases and other infectious processes. While it is not possible to prevent CMV seropositivity in the older KT candidate, it is possible to change CMV prevention protocols to reduce the risk of CMV reactivation after KT.
We also found that the older cohort had a significantly shorter time to CMV viremia and, while not reaching statistical significance (p=0.07), more episodes of CMV reactivation occurred in this group. While the presence of diabetes mellitus, a history of prior transplant and basiliximab induction therapy were associated with CMV viremia in the univariate model, only older age group remained significant in the multivariate analysis.
When applying the 450 IU/ml cut-off, only a history of prior transplantation was significantly associated with the development of CMV viremia in the non-anti-thymoglobulin group. A clinical threshold of CMV viremia has not yet been defined. This is mainly due to a lack of a reference range and viral load values not being comparable between different assays[29,28,33]. We set the threshold for this study at 450 IU/ml because that is the cut-off where treatment for CMV viremia at our institution is initiated. The above-mentioned meta-analysis set their cut-off at 400 IU/ml[22]. One could argue that the results from the different studies are not directly comparable and that the absence of a defined threshold make these results difficult to interpret. Additionally, low-level viremia is often seen in immunocompromised transplant recipients that present with severe infections[34]. For this reason, we included several non-CMV infections syndromes (bacteremia and pneumonia) in our analysis. We did not find a significant association between these infections and CMV viremia in our models. Other authors showed that CMV prophylaxis in solid organ transplant recipients decreases the risk of other infections[35] and that CMV infection increases the risk of subsequent bacterial infection[36], hence preventing CMV reactivation could also diminish the risk of bacterial infections. Although this analysis was outside the scope of our study, these literature findings reinforce the importance of preventing CMV reactivation.
A unique finding of our study is that the first CMV reactivation portends an increased risk for subsequent reactivations, emphasizing the need to avoid that initial episode of CMV reactivation. Taken together, our findings raise several questions. Most importantly, should we use universal prophylaxis in older CMV seropositive kidney transplant recipients given their “high risk” of reactivating CMV and given that avoiding that first CMV reactivation in older adults may be critical. Although very effective in the CMV R+ population[12,17], universal prophylaxis does come with financial as well as non-monetary cost which may be accentuated in the adult patient[37]. For example, a decline in renal function[15,38] and leukopenia which is a relatively common side effect of valganciclovir[39-41], is also a common problem in older KT recipients[41]. Further, the routine addition of valganciclovir to the medical regimen would further contribute to polypharmacy, another source of morbidity.
While our study is limited owing to its single-center, retrospective nature, 12 months follow up and sample size, it does raise important new questions regarding optimal CMV prophylaxis strategies in older KT recipients, a growing subpopulation of KT recipients underrepresented in prior studies.
Future directions should include multicenter trials comparing universal prophylaxis versus pre-emptive therapy or a hybrid model in the older CMV R+ transplant recipient population, as well as immunologic studies to better understand the specific mechanisms that predispose older patients to reactivate CMV [42,43,7]. Finally, frailty[31] and resilience should be included as endpoints of special interest in this patient population, with the aim of avoiding CMV reactivation and improving functional status and outcomes for older adults after KT.
Acknowledgments
Funding: “Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health under award number 5T32AI100851 (MHM, BDA) and National Institute on Aging (NIA) of the National Institutes of Health, Duke Pepper Older Americans Independence Center P30AG028716 (KES). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.” This research was also supported by an internal grant from the Duke Transplant Center (MHM).
Footnotes
Conflict of interests: The authors declare that they have no conflict of interests.
Ethical approval / informed consent: The study was approved by the Duke University Health System Institutional Review Board for Clinical Investigation (Pro00076804). A waiver for informed consent was granted.
References
- 1.Yoshikawa TT, Norman DC (2017) Geriatric Infectious Diseases: Current Concepts on Diagnosis and Management. Journal of the American Geriatrics Society 65 (3):631–641. doi: 10.1111/jgs.14731 [DOI] [PubMed] [Google Scholar]
- 2.Mouton CP, Bazaldua OV, Pierce B, Espino DV (2001) Common infections in older adults. American family physician 63 (2):257–268 [PubMed] [Google Scholar]
- 3.Meier-Kriesche HU, Ojo AO, Hanson JA, Kaplan B (2001) Exponentially increased risk of infectious death in older renal transplant recipients. Kidney international 59 (4):1539–1543. doi: 10.1046/j.1523-1755.2001.0590041539.x [DOI] [PubMed] [Google Scholar]
- 4.Huang E, Segev DL, Rabb H (2009) Kidney transplantation in the elderly. Semin Nephrol 29 (6):621–635. doi: 10.1016/j.semnephrol.2009.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trouillhet I, Benito N, Cervera C, Rivas P, Cofan F, Almela M, Angeles Marcos M, Puig de la Bellacasa J, Pumarola T, Oppenheimer F, Moreno-Camacho A (2005) Influence of age in renal transplant infections: cases and controls study. Transplantation 80 (7):989–992 [DOI] [PubMed] [Google Scholar]
- 6.Adani GL, Baccarani U, Crestale S, Pravisani R, Isola M, Tulissi P, Vallone C, Nappi R, Risaliti A (2019) Kidney Transplantation in Elderly Recipients: A Single-Center Experience. Transplantation proceedings 51 (1):132–135. doi: 10.1016/j.transproceed.2018.04.081 [DOI] [PubMed] [Google Scholar]
- 7.Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, Humar A (2018) The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation 102 (6):900–931. doi: 10.1097/tp.0000000000002191 [DOI] [PubMed] [Google Scholar]
- 8.Opelz G, Dohler B, Ruhenstroth A (2004) Cytomegalovirus prophylaxis and graft outcome in solid organ transplantation: a collaborative transplant study report. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 4 (6):928–936. doi: 10.1111/j.1600-6143.2004.00451.x [DOI] [PubMed] [Google Scholar]
- 9.Bate SL, Dollard SC, Cannon MJ (2010) Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 50 (11):1439–1447. doi: 10.1086/652438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Boven M, van de Kassteele J, Korndewal MJ, van Dorp CH, Kretzschmar M, van der Klis F, de Melker HE, Vossen AC, van Baarle D (2017) Infectious reactivation of cytomegalovirus explaining age- and sex-specific patterns of seroprevalence. PLoS computational biology 13 (9):e1005719–e1005719. doi: 10.1371/journal.pcbi.1005719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kliem V, Fricke L, Wollbrink T, Burg M, Radermacher J, Rohde F (2008) Improvement in long-term renal graft survival due to CMV prophylaxis with oral ganciclovir: results of a randomized clinical trial. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 8 (5):975–983. doi: 10.1111/j.1600-6143.2007.02133.x [DOI] [PubMed] [Google Scholar]
- 12.Witzke O, Hauser IA, Bartels M, Wolf G, Wolters H, Nitschke M (2012) Valganciclovir prophylaxis versus preemptive therapy in cytomegalovirus-positive renal allograft recipients: 1-year results of a randomized clinical trial. Transplantation 93 (1):61–68. doi: 10.1097/TP.0b013e318238dab3 [DOI] [PubMed] [Google Scholar]
- 13.Khoury JA, Storch GA, Bohl DL, Schuessler RM, Torrence SM, Lockwood M, Gaudreault-Keener M, Koch MJ, Miller BW, Hardinger KL, Schnitzler MA, Brennan DC (2006) Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 6 (9):2134–2143. doi: 10.1111/j.1600-6143.2006.01413.x [DOI] [PubMed] [Google Scholar]
- 14.Reischig T, Jindra P, Hes O, Svecova M, Klaboch J, Treska V (2008) Valacyclovir prophylaxis versus preemptive valganciclovir therapy to prevent cytomegalovirus disease after renal transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 8 (1):69–77. doi: 10.1111/j.1600-6143.2007.02031.x [DOI] [PubMed] [Google Scholar]
- 15.Spinner ML, Saab G, Casabar E, Bowman LJ, Storch GA, Brennan DC (2010) Impact of prophylactic versus preemptive valganciclovir on long-term renal allograft outcomes. Transplantation 90 (4):412–418. doi: 10.1097/TP.0b013e3181e81afc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luna E, Caravaca F, Ferreira F, Fernandez N, Martin P, Vargas ML, Saenz de Santamaria J, Garcia Pino G, Azevedo L, Munoz Sanz A (2016) Effect of Cytomegalovirus Infection on Survival of Older Kidney Transplant Patients (D+/R+): Impact of Valganciclovir Prophylaxis Versus Preemptive Therapy. Transplantation proceedings 48 (9):2931–2937. doi: 10.1016/j.transproceed.2016.06.062 [DOI] [PubMed] [Google Scholar]
- 17.Witzke O, Nitschke M, Bartels M, Wolters H, Wolf G, Reinke P, Hauser IA, Alshuth U, Kliem V (2018) Valganciclovir Prophylaxis Versus Preemptive Therapy in Cytomegalovirus-Positive Renal Allograft Recipients: Long-term Results After 7 Years of a Randomized Clinical Trial. Transplantation 102 (5):876–882. doi: 10.1097/tp.0000000000002024 [DOI] [PubMed] [Google Scholar]
- 18.Hibberd PL, Tolkoff-Rubin NE, Conti D, Stuart F, Thistlethwaite JR, Neylan JF, Snydman DR, Freeman R, Lorber MI, Rubin RH (1995) Preemptive ganciclovir therapy to prevent cytomegalovirus disease in cytomegalovirus antibody-positive renal transplant recipients. A randomized controlled trial. Annals of internal medicine 123 (1):18–26 [DOI] [PubMed] [Google Scholar]
- 19.Weclawiak H, Kamar N, Mengelle C, Guitard J, Esposito L, Lavayssiere L, Cointault O, Ribes D, Rostaing L (2008) Cytomegalovirus prophylaxis with valganciclovir in cytomegalovirus-seropositive kidney-transplant patients. Journal of medical virology 80 (7):1228–1232. doi: 10.1002/jmv.21183 [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Ruiz M, Arias M, Campistol JM, Navarro D, Gomez-Huertas E, Gomez-Marquez G, Diaz JM, Hernandez D, Bernal-Blanco G, Cofan F, Jimeno L, Franco-Esteve A, Gonzalez E, Moreso FJ, Gomez-Alamillo C, Mendiluce A, Luna-Huerta E, Aguado JM (2015) Cytomegalovirus prevention strategies in seropositive kidney transplant recipients: an insight into current clinical practice. Transplant international : official journal of the European Society for Organ Transplantation 28 (9):1042–1054. doi: 10.1111/tri.12586 [DOI] [PubMed] [Google Scholar]
- 21.Jung C, Engelmann E, Borner K, Offermann G (2001) Preemptive oral ganciclovir therapy versus prophylaxis to prevent symptomatic cytomegalovirus infection after kidney transplantation. Transplantation proceedings 33 (7-8):3621–3623 [DOI] [PubMed] [Google Scholar]
- 22.Caskurlu H, Karadag FY, Arslan F, Cag Y, Vahaboglu H (2019) Comparison of universal prophylaxis and preemptive approach for cytomegalovirus associated outcome measures in renal transplant patients: A meta-analysis of available data. Transplant infectious disease : an official journal of the Transplantation Society 21 (1):e13016. doi: 10.1111/tid.13016 [DOI] [PubMed] [Google Scholar]
- 23.Hemmersbach-Miller M, Alexander BD, Sudan DL, Pieper C, Schmader KE (2019) Infections after Kidney Transplantation. Does Age Matter? Clinical transplantation:e13516. doi: 10.1111/ctr.13516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horvath MM, Rusincovitch SA, Brinson S, Shang HC, Evans S, Ferranti JM (2014) Modular design, application architecture, and usage of a self-service model for enterprise data delivery: the Duke Enterprise Data Unified Content Explorer (DEDUCE). Journal of biomedical informatics 52:231–242. doi: 10.1016/j.jbi.2014.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 42 (2):377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, Pikis A, Razonable RR, Miller V, Griffiths PD (2016) Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 64 (1):87–91. doi: 10.1093/cid/ciw668 [DOI] [PubMed] [Google Scholar]
- 27.CDC (2017) CDC/NHSN Surveillance Definitions for Specific Types of Infections. https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf.
- 28.Razonable RR, Humar A (2013) Cytomegalovirus in solid organ transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 13 Suppl 4:93–106. doi: 10.1111/ajt.12103 [DOI] [PubMed] [Google Scholar]
- 29.Razonable RR, Humar A (2019) Cytomegalovirus in Solid Organ Transplant Recipients - Guidelines of the American Society of Transplantation Infectious Disease Community of Practice. Clinical transplantation:e13512. doi: 10.1111/ctr.13512 [DOI] [PubMed] [Google Scholar]
- 30.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences 908:244–254 [DOI] [PubMed] [Google Scholar]
- 31.Thomasini RL, Pereira DS, Pereira FSM, Mateo EC, Mota TN, Guimarães GG, Pereira LSM, Lima CX, Teixeira MM, Teixeira ALJ (2017) Aged-associated cytomegalovirus and Epstein-Barr virus reactivation and cytomegalovirus relationship with the frailty syndrome in older women. PloS one 12 (7):e0180841–e0180841. doi: 10.1371/journal.pone.0180841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang GC, Kao WH, Murakami P, Xue QL, Chiou RB, Detrick B, McDyer JF, Semba RD, Casolaro V, Walston JD, Fried LP (2010) Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. American journal of epidemiology 171 (10):1144–1152. doi: 10.1093/aje/kwq062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayden RT, Yan X, Wick MT, Rodriguez AB, Xiong X, Ginocchio CC, Mitchell MJ, Caliendo AM (2012) Factors contributing to variability of quantitative viral PCR results in proficiency testing samples: a multivariate analysis. Journal of clinical microbiology 50 (2):337–345. doi: 10.1128/jcm.01287-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lachance P, Chen J, Featherstone R, Sligl WI (2017) Association Between Cytomegalovirus Reactivation and Clinical Outcomes in Immunocompetent Critically Ill Patients: A Systematic Review and Meta-Analysis. Open Forum Infect Dis 4 (2):ofx029. doi: 10.1093/ofid/ofx029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montoya JG, Giraldo LF, Efron B, Stinson EB, Gamberg P, Hunt S, Giannetti N, Miller J, Remington JS (2001) Infectious complications among 620 consecutive heart transplant patients at Stanford University Medical Center. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 33 (5):629–640. doi: 10.1086/322733 [DOI] [PubMed] [Google Scholar]
- 36.Limaye AP, Bakthavatsalam R, Kim HW, Randolph SE, Halldorson JB, Healey PJ, Kuhr CS, Levy AE, Perkins JD, Reyes JD, Boeckh M (2006) Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation 81 (12):1645–1652. doi: 10.1097/01.tp.0000226071.12562.1a [DOI] [PubMed] [Google Scholar]
- 37.McGillicuddy JW, Weimert NA, Taber DJ, Turner A, Mitchell LA, Wray DW, Egidi MF, Kuppachi S, Hughes MG, Baliga PK, Chavin KD (2010) Can preemptive cytomegalovirus monitoring be as effective as universal prophylaxis when implemented as the standard of care in patients at moderate risk? Transplantation 89 (10):1218–1223. doi: 10.1097/TP.0b013e3181d54ba6 [DOI] [PubMed] [Google Scholar]
- 38.Werzowa J, Schwaiger B, Hecking M, Strassl R, Schmaldienst S, Bohmig GA, Genser B, Saemann MD (2015) Prophylactic CMV therapy does not improve three-yr patient and graft survival compared to preemptive therapy. Clinical transplantation 29 (12):1230–1238. doi: 10.1111/ctr.12657 [DOI] [PubMed] [Google Scholar]
- 39.Brum S, Nolasco F, Sousa J, Ferreira A, Possante M, Pinto JR, Barroso E, Santos JR (2008) Leukopenia in kidney transplant patients with the association of valganciclovir and mycophenolate mofetil. Transplantation proceedings 40 (3):752–754. doi: 10.1016/j.transproceed.2008.02.048 [DOI] [PubMed] [Google Scholar]
- 40.Hwang SD, Lee JH, Lee SW, Kim JK, Kim MJ, Song JH (2018) Effect of Low-Dose Vs Standard-Dose Valganciclovir in the Prevention of Cytomegalovirus Disease in Kidney Transplantation Recipients: A Systemic Review and Meta-Analysis. Transplantation proceedings 50 (8):2473–2478. doi: 10.1016/j.transproceed.2018.01.023 [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Guerra CM, Sumrani N (2018) Effect of Age on Leukopenia Following Renal Transplantation at a Single Center. Progress in transplantation (Aliso Viejo, Calif):1526924818817017. doi: 10.1177/1526924818817017 [DOI] [PubMed] [Google Scholar]
- 42.Kumar D, Mian M, Singer L, Humar A (2017) An Interventional Study Using Cell-Mediated Immunity to Personalize Therapy for Cytomegalovirus Infection After Transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 17 (9):2468–2473. doi: 10.1111/ajt.14347 [DOI] [PubMed] [Google Scholar]
- 43.Lisboa LF, Kumar D, Wilson LE, Humar A (2012) Clinical utility of cytomegalovirus cell-mediated immunity in transplant recipients with cytomegalovirus viremia. Transplantation 93 (2):195–200. doi: 10.1097/TP.0b013e31823c1cd4 [DOI] [PubMed] [Google Scholar]


