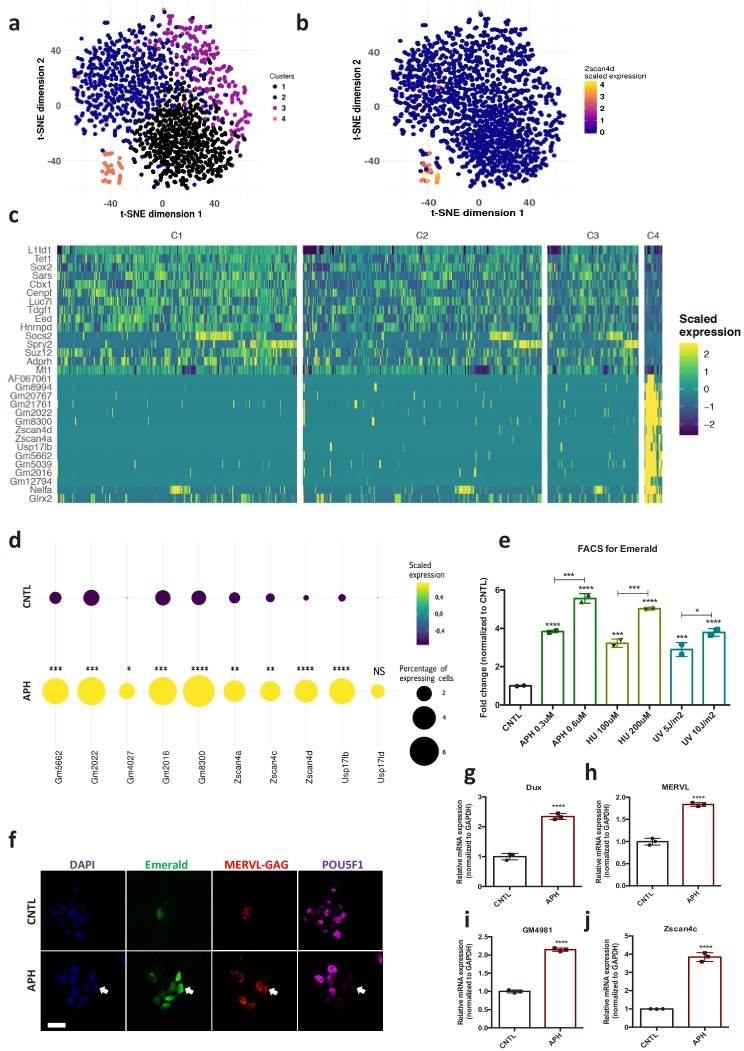
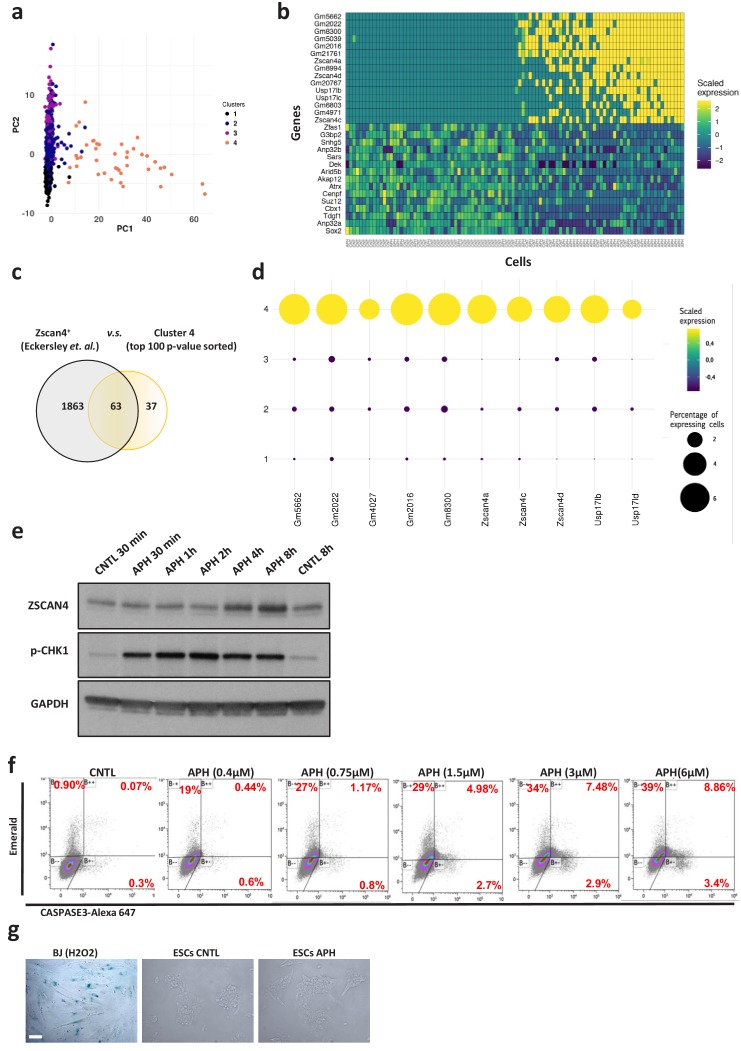
Figure 1. Induction of RS increases the number of 2C-like cells in ESCs culture and activates the expression of 2C-specific genes in mouse embryos.
(a) Clustering of 1399 Drop-seq single-cell expression profiles into four cell populations. The plot shows a two-dimensional representation (t-SNE) of global gene expression relationship; cells are colored according to their cluster. Clusters were identified by shared nearest neighbor algorithm (see methods). (b) t-SNE plot showing Zscan4d expression level across all CNTL and APH-treated cells. (c) Heatmap showing the list of top 30 genes that are differentially expressed between cluster 4 cells (orange cluster in a) and the rest of the population (cluster 1,2, and 3). (d) Plot showing the scaled expression of 2C-specific markers and the percentage of cells expressing 2C-related genes in CNTL and APH-treated condition. Fisher's exact test was used to determine p-values. (e) FACS analysis on pZscan4-Emerald ESCs upon treatment with various RS-inducing agents. (f) Immunostaining of ESCs for ZSCAN4-Emerald, MERVL-GAG and canonical pluripotency marker POU5F1 upon treatment with APH (bar = 25 µm). (g–j) RT-qPCR analysis on blastocyst-stage embryos treated with APH for key 2C-like markers. Statistical significance compared to CNTL unless otherwise indicated. All bar-plots show mean with ± SD (*p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001, one-way ANOVA).