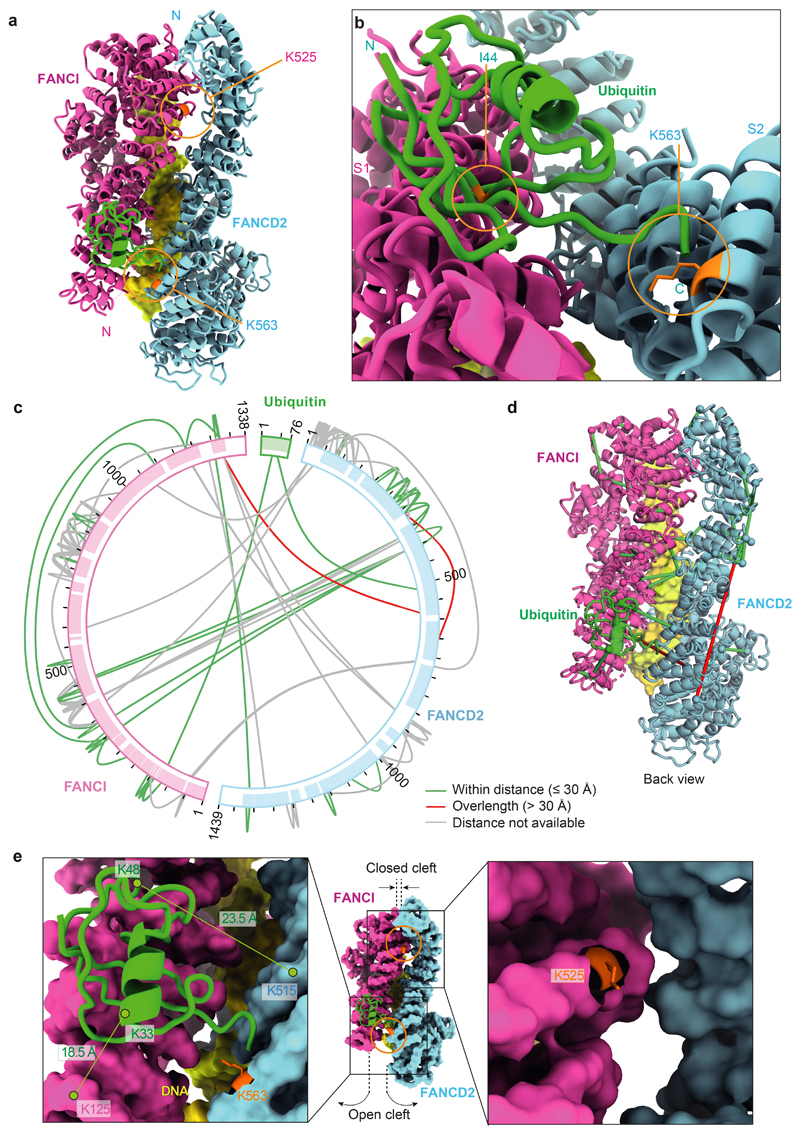
Fig. 2. Ubiquitin is anchored to K563 on FANCD2 but also contacts FANCI.
a The ubiquitin moiety attached to FANCD2 makes extensive contacts with FANCI. b Close-up view of the monoubiquitination site. Ile44 (orange) of ubiquitin is shown. c Map of crosslinks identified in the ubD2–I complex. Crosslinking mass spectrometry revealed 122 crosslinks (1% false discovery rate) between residues that are in close proximity. Crosslinks are colored by Cα-Cα distance between linked residue pairs measured in the 3D model of ubD2–I. Two crosslinks are not compatible with the model but are consistent with the flexibility observed within this complex (Supplementary Video 1). Proteins are shown as curved bars and residues that are present in the 3D model are highlighted. d Crosslinks within expected distance (green) and exceeding expected distance (overlength, red) mapped onto the ubD2–I structure. e Details of lysines K563 in FANCD2 (left) and K525 in FANCI (right) within the ubD2–I structure, shown in sticks for lysines (orange), as a surface representation of the model for FANCD2 (blue) and FANCI (magenta), and in cartoon for ubiquitin (green). The residues crosslinked between ubiquitin and FANCD2 and FANCI are labeled.