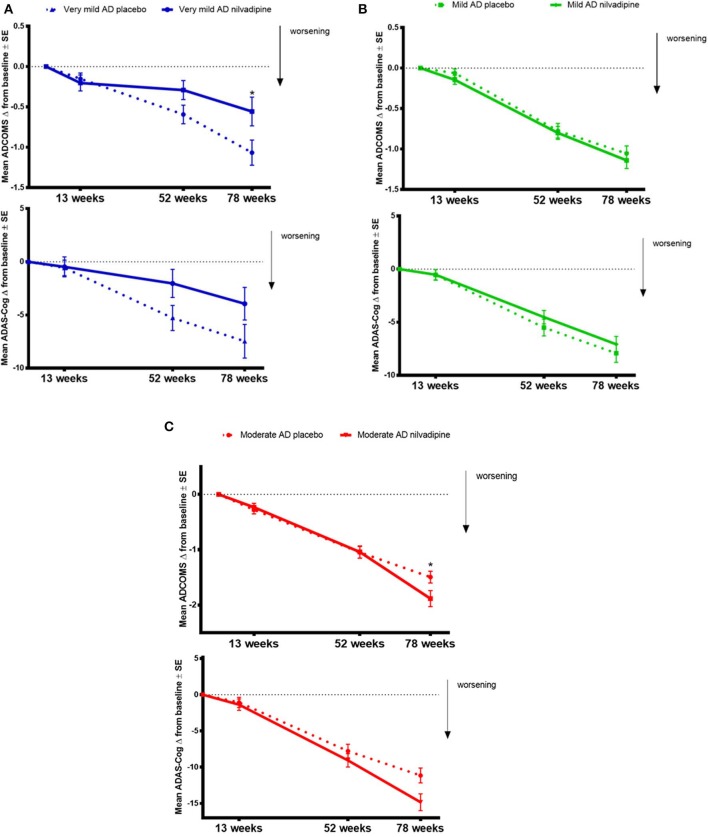
Figure 3.
Data on ADCOMS and ADAS-Cog 12 test. Nilvadipine-treated very mild AD subjects show less cognitive decline compared to controls on the ADCOMS and the ADAS-Cog 12 tests. Mean ± SE [n = 82 for moderate AD (MMSE ≤ 19) on nilvadipine, n = 94 for moderate AD on placebo, n = 118 for mild AD (MMSE 20-24) on nilvadipine, n = 113 for mild AD on placebo, n = 36 for very mild AD (MMSE ≥ 35) on nilvadipine and n = 44] for very mild AD on placebo for the change in ADAS-Cog 12 scores. There was a significant effect for the interaction between treatment, time and baseline AD severity as assessed by MMSE scores after correcting for the confounding effects of APOE, gender and education, p < 0.05. (A) Stratifications show that very mild AD subjects treated with nilvadipine have lower scores on the ADCOMS and the ADAS-Cog 12 compared to placebo after 78 weeks. post-hoc analysis stratified by time show a significant treatment effect at 78 weeks for the ADCOMS. (B) Mild AD subjects treated with nilvadipine scored similarly to their placebo controls on both the ADCOMS and the ADAS-Cog 12 (C). However, moderate AD subjects treated with nilvadipine scored higher on both the ADCOMS and the ADAS-Cog 12 at 78 weeks compared to those on placebo. *p < 0.05.