Abstract
The promise of poly(ADP-ribose) polymerase inhibitors (PARPis) in the management of epithelial ovarian cancer (EOC) is hampered by the limited clinical activity against BRCA wild-type or homologous recombination-proficient EOC. In order to decrease the resistance and increase the efficacy of PARPis, combination treatments of pharmacological ascorbate and PARPis in preclinical BRCA wild-type EOC models were investigated. The cytotoxicity of pharmacological ascorbate, olaparib and veliparib in a panel of BRCA1/2 wild-type EOC cell lines were measured using MTT assays. Poly(ADP-ribose) levels were quantified using chemiluminescent ELISA. The expression of proteins involved in DNA damage and DNA double-strand breaks (DSBs) repair pathways were assessed by western blotting. The in vivo efficacy of pharmacological ascorbate, olaparib and their combination was evaluated in an intraperitoneal xenograft mouse model of BRCA1/2 wild-type EOC. Pharmacological ascorbate induced H2O2-dependent cytotoxicity in BRCA1/2 wild-type EOC cells. SHIN3 and OVCAR5 cells were resistant to olaparib and veliparib treatment; however, the combination of ascorbate with olaparib or veliparib significantly enhanced cell death. Pharmacological ascorbate enhanced the effects olaparib or veliparib by downregulating the expression of BRCA1, BRCA2 and RAD51. Consequently, the combination of pharmacological ascorbate and olaparib potently enhanced DNA DSBs and significantly decreased tumor burden, ascites volume and the number of tumor cells in ascites in mice bearing BRCA1/2 wild-type ovarian cancer xenografts. The combination of pharmacological ascorbate and PARPis may be a promising therapeutic approach worth clinical investigation in patients with BRCA wild-type or PARPi-resistant EOC.
Keywords: ovarian cancer, ascorbate, olaparib, PARP inhibitors, BRCA wild-type
Introduction
Epithelial ovarian cancer (EOC) is the most lethal type of gynecological malignancy and ranks as the 5th leading cause of cancer-associated mortality among women in the USA (1), accounting for 22,240 new cases and 14,070 deaths in 2018 (2). Despite intensive treatment options, including debulking surgery, platinum and taxane-based chemotherapy and targeted therapy, such as poly(ADP-ribose) polymerase inhibitors (PARPis), angiogenesis inhibitors and immunotherapy agents, the overall 5-year survival rate for all types of EOC is relatively low (47.4%) and has remained stagnant for >2 decades (3). Additionally, ~60% of patients with EOC possess distant metastases at initial diagnosis, and the 5-year survival for these patients is considerably lower at 26% (2). There are a number of reasons for these poor survival outcomes, including the absence of reliable and accurate screening tests and limited effectiveness of current chemotherapies (1,2). Cumulative toxicity, cross-resistance to chemotherapies and compromised quality of life are additional serious clinical challenges for patients with EOC. Therefore, developing more effective and less toxic therapeutic strategies that target the fundamental vulnerabilities of EOC is required to improve patients' outcomes and quality of life.
PARPis are a new class of oncology drugs that are transforming the management of EOC (4). PARPis exert anti-cancer properties by trapping PARP on DNA at the sites of single-strand breaks, which leads to DNA repair defects and the generation of DNA DSBs that require homologous recombination (HR) mediated by BRCA1, BRCA2 and other proteins (such as ATM, ATR, RAD51, CHK1 and FANCA) (5–7). Therefore, BRCA1/2 mutant or HR-deficient cells are exceptionally sensitive to PARP inhibition (7–9), and the combination of two genetic deficiencies (e.g., BRCA1/2 and PARP) leads to synthetic lethality in cancer cells. Based on the promising clinical efficacy and the manageable toxicity profile of PARPis in patients with advanced EOC (10–13), three PARPis (olaparib, rucaparib and niraparib) were approved by the U.S. Food and Drug Administration, either as a monotherapy (olaparib and rucaparib) for women with heavily pretreated germline BRCA-mutated (gBRCAm) EOC or as a maintenance therapy (olaparib, rucaparib and niraparib) for women with platinum-sensitive recurrent EOC regardless of BRCA or HR-deficiency (HRD) status. In addition, a recent Phase III multicenter study (NCT01844986) (12) revealed that olaparib maintenance monotherapy significantly improved progression-free survival times in women newly diagnosed with advanced ovarian cancer who harbored a BRCA1/2 mutation (14). However, new challenges have arisen for PARPi therapy. Mutations in BRCA1 or BRCA2 occur in <20% of patients with EOC (16% germline and 4% somatic) (15,16). The majority of patients with EOC are BRCA1/2 wild-type carriers who respond much less favorably to PARPis, limiting the clinical efficacy and utility of PARPis. Additionally, although several clinical studies have shown that some non-gBRCAm or patients with HRD negative cancer can benefit from PARPis (13,17), developing effective prediction tools independent of HRD is difficult, due to the lack of predictive biomarkers, which makes patient selection challenging. The combination of a PARPis and chemotherapy have yet to show significant clinical benefits, and enhanced myelosuppression, as the main dose-limiting toxicity, has been observed (18,19), which may limit future combinatorial use of these two types of therapy. As such, developing novel PARPi combination therapies with a broad efficacy and low toxicity is one potential direction for improving treatment of patients with EOC.
Previously, it has been shown that ascorbate (vitamin C) when used in high intravenous doses (IVC), has potential as a therapeutic agent for the treatment of a variety of different types of cancer (20–25). High-dose IVC, in contrast to oral doses, establishes pharmacological concentrations in the millimolar range in tissues, and selectively kills cancer cells by generating H2O2 in the extracellular fluid, while leaving healthy cells unharmed (21–23). The exquisite selectivity of pharmacological ascorbate suggests a low toxicity of IVC treatment. Multiple early phase clinical trials in patients with solid or hematological malignancies, where IVC was used alone or in combination with conventional chemotherapies or radiation therapy, demonstrated that IVC was safe, well tolerated and did not increase the toxicities of standard therapies (20,25–28). The authors of the present study first demonstrated a notable decrease in chemo-associated toxicities by adding IVC to standard carboplatin/paclitaxel chemotherapy in patients with stage III or IV EOC (20). In addition, the preliminary clinical benefits in prolonged relapse time and/or tumor responses by adding IVC to standard chemo- or radiation therapy has been demonstrated (20,25).
By generating H2O2, pharmacological ascorbate damages DNA and preferentially kills cancer cells (20,29). Therefore, it was hypothesized that the combination of pharmacological ascorbate and PARPis may enhance DNA repair deficiency, and thus enhance the therapeutic effect of either agent alone against EOC, regardless of BRCA status. In the present study, the DNA damage response (DDR) induced by pharmacological ascorbate in ovarian cancer cells bearing wild-type BRCA1/2 was characterized, and the efficacy and feasibility of the combination treatment of pharmacological ascorbate and the PARPi, olaparib, in preclinical models of EOC harboring wild-type BRCA were investigated.
Materials and methods
Cell culture and reagents
Human EOC cell lines OVCAR8 and SHIN3 were kindly provided by Dr Peter Eck (University of Manitoba, Manitoba, Canada) and OVCAR3, OVCAR5, OVCAR10, SKOV3, A2780 and HIO-80 (an immortalized, nontumorigenic human ovarian epithelium cell line) were kindly provided by Dr Thomas Hamilton, or were derived by Dr Andrew K. Godwin, both of the Fox Chase Cancer Center (Philadelphia, USA). SHIN3 cells were maintained in DMEM supplemented with 10% FBS, (both Sigma-Aldrich; Merck KGaA) and 1% penicillin-streptomycin. HIO-80 was cultured in M199/MCDB105 medium (1:1, v/v) containing 4% FBS, insulin (0.3 U/ml) and 2 mM L-glutamine. The remaining cell lines were cultured in PRMI-1640 medium supplemented with 10% FBS and 1% penicillin-streptomycin. All cells were cultured at 37°C with 5% CO2 and 85–95% humidity. Cell line authentication was carried out by the Clinical Molecular Oncology Laboratory of University of Kansas Medical Center (Kansas, USA) using multiplex short tandem repeat DNA profiling.
L-Ascorbic Acid (Thermo Fisher Scientific, Inc.) was prepared as 1 M stock solutions in sterile water, with sodium hydroxide added drop-wise to adjust the pH to 7.0. Aliquots were stored at −80°C and thawed for single use. Catalase (Sigma-Aldrich; Merck KGaA) was prepared in distilled water at 10,000 units/ml, and was used at a working concentration of 600 units/ml. Olaparib and veliparib were obtained from Selleck Chemicals and were prepared in dimethyl sulfoxide (DMSO) and diluted with cell culture media to working concentrations, for the in vitro experiments. For the in vivo experiments, olaparib was dissolved in PBS containing 10% 2-hydroxy-propyl-betacyclodextrin (Sigma-Aldrich; Merck KGaA). All other reagents and chemicals were obtained from Thermo Fisher Scientific, Inc., unless specifically indicated.
BRCA1/2 mutation analysis
The BRCA1/2 wild-type status was reported previously (30) for all the EOC cell lines used in the present study except SHIN3. The genomic DNA of SHIN3 cells was extracted using a Blood & Cell Culture DNA Mini kit (Qiagen GmbH). The largest and functionally most important exon (exon 11) of both BRCA1 (3,630 bp) and BRCA2 (5,018 bp) was amplified from the genomic DNA template using PCR as previously described (31). The PCR amplicons were submitted to Genewiz, Inc. for DNA sequencing. The primer sequences are provided in Table SI. The thermocycling conditions and Taq enzyme used were as previously described (31). DNA sequences were analyzed using the DNASTAR analysis package (version 8.1; DNASTAR, Inc.). Both nucleic acid and amino acid sequences were aligned using BioEdit (version 7.2) (32).
MTT assay
Cells were seeded at a density of 1×104 cells per well in a 96 well plate, and incubated overnight. Cells were then exposed to a serial dilution of ascorbate (0–3.5 mM), olaparib (0–1,000 µM in SHIN3 cells; 0–800 µM in OVCAR5 cells) and veliparib (0–1,000 µM in SHIN3 cells; 0–800 µM in OVCAR5 cells), or treatment combinations and incubated for 24 or 48 h. In the drug combination groups, either olaparib or veliparib was added 15 min prior to ascorbate treatment. Following treatment, the culture medium was replaced with fresh, drug-free medium, and cells were incubated with MTT for 4 h. Formazan crystals were dissolved using DMSO and the absorbance at 492 nm was measured on a Synergy™ 4 Hybrid microplate reader (BioTek Instruments, Inc.). The half maximal inhibitory concentration (IC50) was determined using a non-linear regression analysis to fit the data to the log10 [inhibitor] compared with a normalized response with a variable slope model.
Different concentrations of ascorbate (ranging from 0–5 mM) were used to avoid drawing conclusions from a single particular concentration. Concentration at IC50 or a concentration range including the IC50 were used. If the treatment time was <48 h, concentrations >IC50 were used, with additional multiple concentrations including at least one close to or lower than the IC50. The in vitro concentration ranges used in the present study are easily achievable in patients by intravenous ascorbate infusion (26).
Poly(ADP-ribose) (PAR) level measurement
PAR levels were measured using a HT PARP in vivo Pharmacodynamic assay II (Trevigen, Inc.), and normalized to the protein contents. Protein concentrations of cell lysates were measured using a Pierce bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.).
Western blot analysis
Cells were lysed in ice-cold radioimmunoprecipitation buffer (Thermo Fisher Scientific, Inc.), supplemented with cOmplete™ Mini Protease Inhibitor Cocktail Tablets (Sigma-Aldrich, Merck KGaA) and Halt™ Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific, Inc.). Protein concentration was determined using the Bradford Protein Assay Kit (Bio-Rad, Inc.). A total of 60 µg protein/lane was resolved on the 4–20% Mini-PROTEAN TGX™ Precast gels (Bio-Rad, Inc.) and transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Inc.). The membranes were blocked using 5% skim milk in TBST (20 mM Tris_HCl, pH 7.4, 150 mM NaCl, 0.1% Tween 20) for 1 h at 4°C, followed by incubation at 4°C overnight with specific antibodies against H2AX (1:500; Cell Signaling Technology, Inc.; cat. no. 7631); p-H2AXSer139 (1:1,000; Cell Signaling Technology, Inc.; cat no. 9718); ATM (1:1,000; Cell Signaling Technology, Inc.; cat. no. 2873); p-ATMSer1981 (1:500; Cell Signaling Technology, Inc.; cat. no. 13050); BRCA1 (1:1,000; Cell Signaling Technology, Inc.; cat. no. 14823); BRCA2 (1:1,000; R&D Systems, Inc.; cat. no. MAB2476); RAD51 (1:1,000; Cell Signaling Technology, Inc.; cat. no. 8875); Ku70 (1:1,000; Cell Signaling Technology, Inc.; cat. no. 4588); Ku80 (1:1,000; Cell Signaling Technology, Inc.; cat. no. 2,180); p-DNA-PKcsThr2609 (1:350; Thermo Fisher Scientific, Inc.; cat. no. PA5-12913); DNA-PKcs (1:4,000; Santa Cruz; cat. no. sc-9051); β-actin (1:5,000; Thermo Fisher Scientific, Inc.; cat. no. MA5-15739); or vinculin (1:1,000; Cell Signaling Technology, Inc.; cat. no. 13901). Target proteins were visualized using horseradish peroxidase (HRP)-conjugated goat-anti-rabbit IgG (1:5,000; Cell Signaling Technology, Inc.; cat. no. 7,074) or HRP-conjugated horse-anti-mouse IgG (1:5,000; Cell Signaling Technology, Inc.; cat. no. 7076) for 1 h at room temperature with Pierce™ ECL Plus Western blotting substrate (Thermo Fisher Scientific, Inc.). Each western blot analysis was performed. b-actin or vinculin were used as the loading controls. In vivo xenograft mouse model. All procedures were performed in accordance with a protocol (ACUP #2018-2443) approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center (Kansas, USA). The intraperitoneal (i.p.) tumor xenografts were established via i.p. injection of 2×106 SHIN3 cells suspended in 200 µl PBS in fifty 4–6-week-old female athymic NCr-nu/nu mice (20–25 g body weight; National Cancer Institute). A total of 2 weeks after cell injection, mice were randomly grouped as follows: i) Control group; i.p. injection of saline solution osmotically equivalent to ascorbate twice daily and olaparib's solvent (PBS containing 10% 2-hydroxy-propyl-betacyclodextrin) once daily in volumes equivalent to the olaparib treated group; ii) ascorbate group, i.p. injection of ascorbate at 4 g/kg twice daily; iii) olaparib group, i.p. injection of olaparib at 50 mg/kg once daily; and iv) combination of ascorbate and olaparib, which were prepared and administered in the same manner as individual drug treatments. After 25 days of treatment, all mice were euthanized by CO2 inhalation in a closed chamber (20% volume/min) followed by bilateral thoracotomy as approved in the protocol, and gross necropsy was performed with tumor weights and ascites volumes measured, and the number of tumor cells in the ascetic fluids counted. The total tumor burden of each mouse was indicated as total tumor weight at the end of experiment. The liver, kidney and spleen from each group were subjected to histopathological analysis using hematoxylin and eosin (H&E) staining, on 4-µm tissue sections with an automated procedure, as previously reported (33).
Statistical analysis
All statistical analyses were performed using GraphPad Prism version 5.0 (GraphPad Software, Inc.). Multiple comparisons between groups were performed using a one-way ANOVA with a post-hoc Turkey's test with a family-wise error rate of 0.05. Adjusted P<0.05 was considered to indicate a statistically significant difference.
Results
Pharmacological ascorbate induces H2O2-dependent cytotoxicity in BRCA1/2 wild-type EOC cells
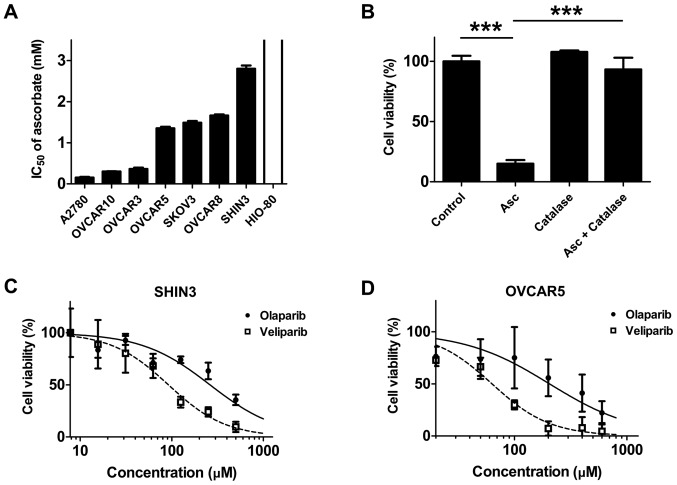
Exon 11 is the largest and most functionally important exon. To the best of our knowledge, the current study was the first to sequence the BRCA1 and 2 genes (both of exon 11) of SHIN3 cells. As indicated in Fig. S1, a single variant of A→G at codon 349 of BRCA1 was detected, which is not known to have a functional outcome or be associated with breast or ovarian cancer. In addition, two variants were detected at codon 2660 A→G and codon 4560 G→C, in exon 11 of BRCA2, which do not result in amino acid changes. The present results indicate that no functional mutations were detected in exon 11 of BRCA1 and 2 in SHIN3 cells. A panel of BRCA1/2 wild-type human ovarian cancer cell lines (A2780, OVCAR10, OVCAR3, OVCAR5, SKOV3, OVCAR8 and SHIN3) (34), and an immortalized, non-tumorigenic human ovarian epithelium cell line (HIO-80) were then screened for sensitivity to ascorbate. As presented in Fig. 1A and Table SII, the IC50 values of ascorbate in the ovarian cancer cells ranged from 0.15–2.80 mM, which is readily achievable by i.v. ascorbate infusion (23,26). In contrast, HIO-80 cells were resistant to ascorbate treatment in the tested concentration range (0.0–3.5 mM), with an IC50 value >3.5 mM (Fig. 1A). When catalase, a H2O2 scavenger, was added to the culture medium, SHIN3 cells were protected from the cytotoxic effects of ascorbate (P<0.001; Fig. 1B), suggesting that the ascorbate cytotoxicity was mediated through H2O2, consistent with previously published studies (21,23).
Figure 1.
Cytotoxicity of pharmacological ascorbate and PARPis olaparib and veliparib in human BRCA1/2 wild-type EOC cells. (A) IC50 values of pharmacological ascorbate across a panel of human BRCA1/2 wild-type EOC cell lines and the non-tumorigenic human ovarian epithelium cell line, HIO-80. Cells were treated with a range of ascorbate concentrations from 0–3.5 mM for 48 h, before the viability was assessed. The IC50 was defined as the concentration of drug that inhibited cell growth by 50% relative to the vehicle control. Data are presented as the mean ± standard error of two to five independent experiments (n=3), and is detailed in Table SII. HIO-80 cells are resistant to ascorbate and the IC50 value of ascorbate in HIO-80 cells was not reached within the tested concentration range. (B) Catalase (100 U/ml) reversed the cytotoxicity induced by 3.5 mM ascorbate. Data are presented as the mean ± standard deviation of three independent experiments. ***P<0.001. (C) Cell growth inhibition curves of SHIN3 cells treated with olaparib and veliparib. (D) Cell growth inhibition curves of OVCAR5 cells treated with olaparib and veliparib. Data are presented as the mean ± standard deviation of three independent experiments. The IC50 values in both SHIN3 and OVCAR5 cells treated with olaparib and veliparib are detailed in Table SII. EOC, epithelial ovarian cancer; PARPi, poly(ADP-ribose) polymerase inhibitor; Asc, ascorbate.
The cell lines exhibiting the highest levels of resistance to ascorbate (SHIN3) and a moderately resistant cell line OVCAR5 were selected as representative cell lines, and their sensitivity to the PARPis olaparib and veliparib. In agreement with their BRCA wild-type status, both SHIN3 and OVCAR5 cells exhibited resistance to the PARPi treatments, as the concentrations of PARP required to exert inhibitory effects on cell viability were particularly high (35) (Fig. 1C and D; Table SII).
Pharmacological ascorbate in combination with PARPis synergistically inhibits the growth of BRCA1/2 wild-type EOC cells
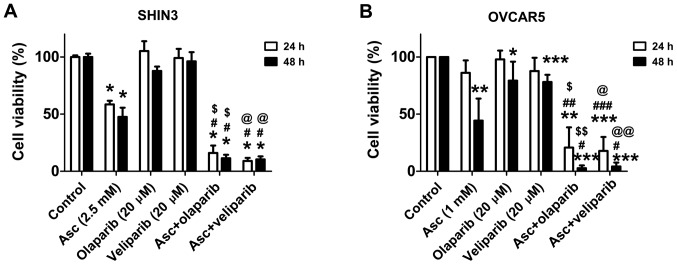
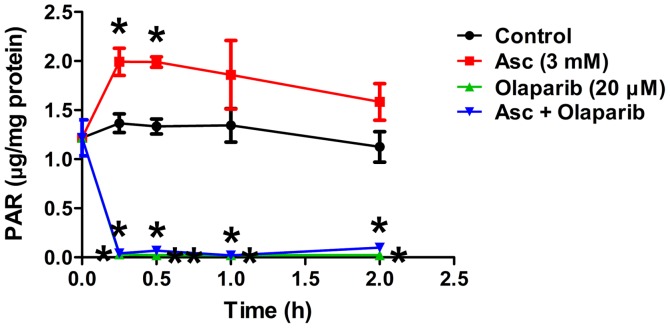
Since ascorbate-induced H2O2 damage to DNA in EOC cells (20) and PARPis impair DNA damage repair, it was hypothesized that the combination of pharmacological ascorbate and PARPis may enhance DNA repair deficiency and improve therapeutic efficacy against EOC. In order to verify this hypothesis, the effects of the combination treatment of pharmacological ascorbate and PARPi olaparib or veliparib in SHIN3 and OVCAR5 cells were determined. Treatment with olaparib (20 µM) or veliparib (20 µM) for 24 and 48 h, respectively, minimally affected the cell viability of SHIN3 and OVCAR5 (Fig. 2A and B). The combined treatment of pharmacological ascorbate with either olaparib or veliparib significantly decreased cell viability compared with either single drug treatment and vehicle control, in both SHIN3 and OVCAR5 cells (Fig. 2A and B). PAR levels were significantly increased by pharmacological ascorbate in SHIN3 cells compared with the control, as early as 15 min (P<0.05; Fig. 3), suggesting that PARP was activated as a cellular response to the H2O2-induced DNA damage mediated by ascorbate. Treatment with olaparib significantly decreased PAR levels in the presence of ascorbate compared with the control (P<0.05; Fig. 3), suggesting that PARP-mediated DNA repair was inhibited. Taken together, these results suggest that when PARP activity is inhibited, pharmacological ascorbate significantly potentiates cell death, potentially through enhanced DNA damage in BRCA1/2 wild-type EOC cells.
Figure 2.
Combining pharmacological ascorbate and PARPis synergistically inhibits the viability of BRCA1/2 wild-type EOC cells. Cell viability of (A) SHIN3 (5 independent experiments each performed in triplicate) or (B) OVCAR cells (4 independent experiments each performed in triplicate) following treatment with vehicle, 2.5 mM ascorbate, 20 µM olaparib, 20 µM veliparib, ascorbate + olaparib or ascorbate + veliparib for 24 and 48 h. Data are expressed as the mean ± standard deviation. *P<0.05, **P<0.01, ***P<0.001 vs. control; #P<0.05, ##P<0.01, ###P<0.001 vs. Asc; $P<0.05, $$P<0.01 vs. olaparib; @P<0.05, @@P<0.01 vs. veliparib. EOC, epithelial ovarian cancer; PARPi, poly(ADP-ribose) polymerase inhibitor; Asc, pharmacological ascorbate.
Figure 3.
Olaparib potently inhibits PARP activity and the inhibition cannot be rescued by pharmacological ascorbate. SHIN3 cells were treated with vehicle, 3 mM Asc, 20 µM olaparib or a combination of ascorbate and olaparib. PAR levels were measured and normalized to the protein concentration. *P<0.01, **P<0.001 vs. control at corresponding time. PARP, poly(ADP-ribose) polymerase; PAR, poly(ADP-ribose); Asc, ascorbate.
Pharmacological ascorbate inhibits HR repair of DNA DSBs in BRCA1/2 wild-type EOC cells
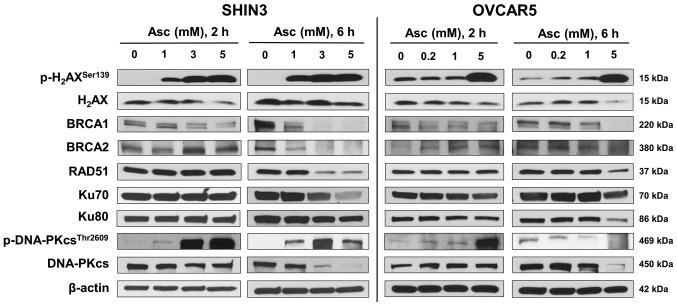
As pharmacological ascorbate induced H2O2 and caused DNA damage in cancer cells (20), the effects of pharmacological ascorbate on DDR in BRCA1/2 wild-type EOC cells was determined in the present study, with a focus on the HR and non-homologous end joining (NHEJ) signaling pathways. Treatment with pharmacological ascorbate for 2 and 6 h both notably increased p-H2AXSer139 levels in both SHIN3 and OVCAR5 cells, a marker of DNA DSBs (Fig. 4). Expression of BRCA1 was downregulated in both tested cell lines 2 h post-ascorbate treatment and further decreased 6 h post treatment (Fig. 4). Notably, the expression of BRCA2 and RAD51 was slightly increased in SHIN3 cells 2 h post treatment, followed by decreases 6 h post treatment (Fig. 4), suggesting that pharmacological ascorbate transiently activated HR repair machinery as part of the stress response, and then inhibited the HR repair machinery. Similar patterns of changes in the expression of BRCA2 and RAD51 were observed in OVCAR5 cells (Fig. 4). Together, these data suggest that pharmacological ascorbate inhibited HR DNA DSBs repair pathway by decreasing the expression of BRCA1, BRCA2 and RAD51 in BRCA1/2 wild-type EOC cells.
Figure 4.
Pharmacological ascorbate induced DNA double stranded breaks and inhibited homologous repair in BRCA1/2 wild-type epithelial ovarian cancer cells, while regulating non-homologues end joining repair in a cell line-dependent manner. SHIN3 and OVCAR5 cells were treated with Asc for either 2 or 6 h. Representative western blots from two independent experiments are presented. β-actin served as a loading control. Asc, ascorbate.
The NHEJ pathway was also investigated. The data revealed that pharmacological ascorbate treatment minimally affected the expression of Ku70 and Ku80 in SHIN3 cells within the first 2 h. After 6 h of treatment, a dose-dependent decrease in the expression of Ku70 and Ku80 was observed following ascorbate treatment (Fig. 4). Ascorbate treatment also decreased the expression of Ku70 and Ku80 in a dose-dependent manner in OVCAR5 cells at either 2 or 6 h of treatment (Fig. 4). Ascorbate treatment increased the levels of p-DNA-PKcsThr2609, a product of activated NHEJ, in SHIN3 and OVCAR5 cells after of 2 h treatment as part of the stress responses. After 6 h, p-DNA-PKcsThr2609 levels were decreased in OVCAR5 cells, but not in SHIN3 cells (Fig. 4). However, the expression of total DNA-PKcs was decreased by ascorbate in a dose-dependent manner in SHIN3 cells after both 2 and 6 h treatment. In OVCAR5 cells DNA-PKcs was first upregulated (2 h) and then downregulated (6 h) following ascorbate treatment (Fig. 4). These data suggest that the regulation of pharmacological ascorbate on the NHEJ DNA repair proteins (Ku70, Ku80 and DNA-PKcs) was cell-line dependent with an overall tendency of inhibition.
Combination of pharmacological ascorbate and olaparib enhances DNA DSBs
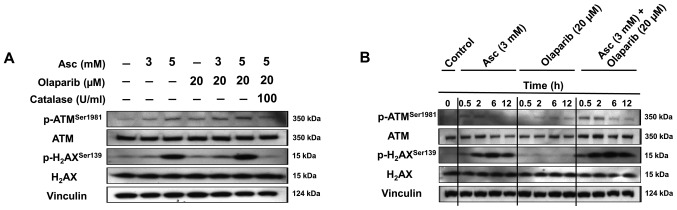
As presented in Fig. 5A, treatment with ascorbate alone or olaparib alone increased the expression of p-ATMSer1981, an early marker of oxidative DNA damage, and the expression of p-H2AXSer139, a marker of DNA DSBs, in a dose-dependent manner in SHIN3 cells treated for 2 h. The combination treatment of ascorbate and olaparib further enhanced the expression of p-ATMSer1981 and p-H2AXSer139 compared with both drugs alone. Treatment with catalase decreased the levels of p-ATMSer1981 and p-H2AXSer139 when it was added prior to treatment with olaparib and ascorbate, suggesting that catalase protects cells from oxidative DNA damage. Similar observations were also observed in OVCAR5 cells (Fig. S2).
Figure 5.
Combination treatment of pharmacological ascorbate and olaparib enhanced DNA double stranded breaks. (A) Representative western blotting images presenting treatment with Asc in combination with olaparib leads to enhanced expression of p-ATMSer1981 and p-H2AXSer139 compared with either treatment alone in SHIN3 cells. Cells were treated with the drug combinations at the indicated concentrations for 2 h. (B) Representative western blotting images demonstrate the time course of p-ATMSer1981 and p-H2AXSer139 levels with Asc + olaparib, respectively, in SHIN3 cells. Cells treated with the different drug combinations at the indicated concentrations were collected at the indicated time points. Experiments were performed twice independently. Vinculin served as a loading control. Asc, ascorbate.
In addition, the time-dependent expression levels of p-ATMSer1981 and p-H2AXSer139 were detected following incubation with 3 mM ascorbate in SHIN3 cells (Fig. 5B). Consistently, the combination treatment of ascorbate and olaparib further increased the expression of p-ATMSer1981 and p-H2AXSer139 compared with either single agent treatment alone at the same time points (Fig. 5B).
Combination treatment of pharmacological ascorbate and olaparib significantly decreases tumor and ascites burden in vivo
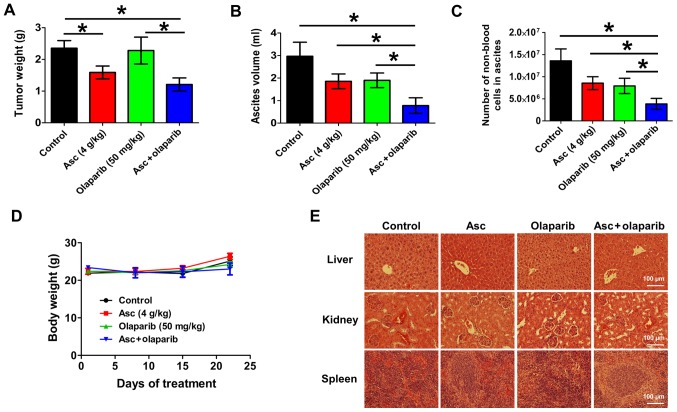
In order to assess the therapeutic efficacy of combination pharmacological ascorbate and olaparib in vivo, a validated xenograft mouse model of ovarian cancer was used (20). This model mimics the later stages of EOC with clinical observations of abdominal tumor dissemination and ascites formation in patients. After 25 days of treatment, ascorbate (4 g/kg) alone significantly decreased tumor weight by 32.5% compared with the vehicle control (P<0.05; Fig. 6A). Olaparib (50 mg/kg) alone had minimal effects (P>0.05) on the tumor weight of these BRCA wild-type xenografts (Fig. 6A). The combination treatment of ascorbate and olaparib significantly decreased tumor weight by 48.6% compared with the vehicle control (P<0.05; Fig. 6A). Treatment with ascorbate or olaparib alone did not significantly decrease the ascites volumes or number of tumor cells in the ascites at the end of treatment, the combination treatment resulted in a 73.8% decrease in ascites volume (P<0.05; Fig. 6B) and a 71.7% decrease in the number of tumor cells in the ascetic fluids (P<0.05; Fig. 6C) relative to the vehicle control. The decrease was significant compared with either treatment alone. All treatments were well tolerated and did not result in weight loss (Fig. 6D). H&E staining demonstrated no pathological changes in the livers, kidneys or spleens of the animals in all treatment groups, suggesting that ascorbate, olaparib and the combination treatment were of low toxicity at the tested concentrations (Fig. 6E).
Figure 6.
Combination treatment of pharmacological ascorbate and olaparib decreases tumor burden, ascites volume and the non-blood cells number in ascites in vivo. (A) Tumor weights, (B) ascites volume and (C) number of non-blood cells in ascites in the xenograft mouse model treated with vehicle. Asc (4 g/kg i.p., twice daily), olaparib (50 mg/kg, i.p., once daily) or the combination treatment for 25 days. *P<0.05. (D) Body weights of mice throughout the treatment period. Data are presented as the mean ± standard error of mean. (E) Representative hematoxylin and eosin stains of liver, kidney and spleen tissues from each treatment group. Scale bar, 100 µm. Asc, ascorbate; i.p. intraperitoneal.
Discussion
BRCA1/2 mutations occur in <20% of patients with EOC, and it is hypothesized that this mutation limits the clinical efficacy of PARPis (15,16). However, PARPis are used in certain patients with EOC regardless of BRCA status (36,37). Currently, three PARPis are used as standard treatment for EOC, and two of these, olaparib and rucaparib, are used for treatment of recurrent BRCA mutant ovarian cancer, as well as maintenance therapy in platinum-sensitive relapsed EOC regardless of BRCA or HRD status (36,37). Niraparib, the third clinically used PARPi, is indicated for maintenance, and used irrespective of BRCA status (38). There is an unmet clinical need to decrease the resistance and enhance the efficacy of PARPis.
In order to expand the applicability and increase the efficacy of PARPis, the addition of pharmacological ascorbate to treatment with PARPis in BRCA wild-type EOC models was assessed for several reasons: i) A previous study demonstrated that pharmacological ascorbate decreased the toxicities of conventional chemotherapies (20); ii) pharmacological ascorbate produces peroxide and damages DNA, thus could work synergistically with an inhibitor of the DNA repair machinery, as presented in the present study; and iii) ascorbate influenced the homologous recombination pathway, and thus could decrease resistance to PARPis, also demonstrated in the present study. Therefore, the present study highlights the potential of combining pharmacological ascorbate and PARPis and how it may benefit a broader population of patients with EOC, even with wild-type BRCA. A clinical proof-of-concept study is required as the next step to further investigate this potential.
The present study demonstrates the preclinical efficacy and feasibility of combining pharmacological ascorbate and PARPis for treating BRCA wild-type ovarian cancer. In vitro, the combination synergistically induced the death of BRCA wild-type EOC cells; in vivo, the combination treatment significantly decreased tumor and ascites burdens without inducing any toxicity. The present study demonstrates that pharmacological ascorbate potentiates the therapeutic efficacy of olaparib in BRCA wild-type EOC by inducing HR deficiency or a ‘BRCAness’ phenotype. Therefore, the combination used in the present study is a novel and potentially promising therapeutic option for treating patients with EOC, particularly those who do not respond to PARPis alone. Such a strategy could be applied to a variety of heterogeneous and hard-to-treat malignancies, including breast, pancreatic and prostate cancer, where BRCA1, BRCA2 or other HR repair proteins are instrumental in the repair of DNA DSBs and the potential of PARPis has not yet been fully exploited (39).
Consistent with previous studies (22,29,40), the data in the present study demonstrated that treatment with pharmacological ascorbate resulted in the production of H2O2, which damages DNA, leading to PARP activation, and this was impaired by the PARPis. The oxidative stress induced by pharmacological ascorbate caused excessive DNA DSBs in BRCA1/2 wild-type EOC cells within the first 6 h of treatment. The concentration and time ranges are clinically relevant to those of i.v. ascorbate infusion (26). Pharmacological ascorbate is selectively lethal to cancer cells, but not normal cells, which is partially attributed to the increased intracellular levels of reactive oxygen species (ROS) and the decreased ability to metabolize H2O2 in cancer cells compared with normal cells (41,42). The ROS-induction mechanism of high dose ascorbate provides the first rationale for combining ascorbate with a PARPi.
Ascorbate inhibited DNA repair enzymes, which provides another rationale for combined treatment with PARPi. HR and NHEJ are the two primary DNA DSBs repair pathways in eukaryotic cells (43). HR is a template-directed DNA repair with high-fidelity, which is crucial for the maintenance of both telomere integrity and genomic stability; whereas NHEJ is an error-prone DNA repair process, which does not use a complementary template and can introduce deleterious mutations during repair (43). The results of the present study suggest that pharmacological ascorbate suppressed the expression of HR repair proteins BRCA1, BRCA2 and RAD51, leading to HR deficiency. In addition, ascorbate influences the NHEJ pathway, impeding both HR and NHEJ pathways. Patel et al (44) and Do et al (45) reported that with PARP inhibition, HR-deficient cancer cells upregulated the NHEJ as an alternative DNA repair pathway. As pharmacological ascorbate impedes both HR and NHEJ, adding it to a PARPi can further promote genomic instability and enhance cytotoxicity.
Patients with EOC with wild-type BRCA are considerably less responsive to PARPis compared with carriers of germline or somatic BRCA mutations (13,17,46). Consistent with the clinical observations, the present study demonstrated that BRCA wild-type EOC cells are not sensitive to olaparib, both in vitro and in vivo. Addition of pharmacological ascorbate to olaparib overcomes the resistance to olaparib and significantly decreased tumor burden. These results highlight the potential of a novel clinical solution for patients with EOC who do not benefit from PARPis alone.
It is well-documented that high-dose IVC is well tolerated with minimal toxicity in humans (26–28,47). A previous Phase I/IIa clinical trials in patients with EOC (20) and pancreatic cancer (40), together with other trials (25,27,28), have consistently demonstrated that adding IVC to standard treatments (chemotherapy or radiation therapy) is safe, well tolerated, feasible and potentially effective. Addition of IVC to carboplatin and paclitaxel chemotherapy substantially decreased side-effects in patients with stage III or IV EOC (20). Potential survival benefits of IVC were reported in combination with chemotherapy or radio-chemotherapy in the treatment of pancreatic cancer, glioblastoma multiforme and advanced-stage non-small cell lung cancer (25,28). Several randomized Phase II trials evaluating the efficacy of IVC are underway (48).
A limitation of the present study lies in the cell line-derived xenograft, which may not be representative of the heterogeneous nature of tumors in patients. Due to the idiosyncratic characteristics of different tumors from different patients, the present study now warrant testing this novel drug combination in multiple patient-derived xenograft mouse models, which will provide more relevant data for future clinical trials. Nevertheless, the results of the present study highlight new opportunities for the translational studies of pharmacological ascorbate in combination with PARPis for treating patients with EOC, regardless of BRCA or HRD status.
In conclusion, the present study demonstrated that the combination treatment of pharmacological ascorbate with PARPis had the potential to provide therapeutic benefits to patients with ovarian cancer who do not respond to PARPis alone. The advantages of this combination therapy lie in the potentially broad applicability, improved efficacy and low toxicity.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Peter Eck (University of Manitoba; Manitoba, Canada) for providing the SHIN3 cell line, Ms. Min Yang (University of Kansas Medical Center; Kansas City, USA) for helping to generate part of the western blotting data, Dr. Kishore Polireddy and Dr. Thuy-Vy Do (University of Kansas Medical Center; Kansas City, USA) for assisting with exploratory experiments, Dr. Chunhua Li and Dr. Nan He (University of Kansas Medical Center; Kansas City, USA) for DNA sequencing and sequence analysis, and the staff of the Clinical Molecular Oncology Laboratory of University of Kansas Medical Center for cell line authentication.
Funding
The present study was financially supported by a bridging grant from the University of Kansas Research Institute (Kansas, USA), and a grant from the University of Kansas Endowment provided by the GR's Foundation, Mosby Lincoln Foundation, and Donlan Foundation (Kansas, USA).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
QC, AKG, JAD and YM conceived and designed the study. YM, PC and DK developed the methodology, performed the experiments and collected data. YM, PC, QC and DK analyzed, computed and interpreted the data. YM wrote the manuscript. YM, QC, AKG, JAD, PC and DK reviewed and revised the manuscript. JAD, AKG and QC provided administrative, technical and material support. QC supervised the study.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A, Siegel RL. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.https://seer.cancer.gov/statfacts/html/ovary.html [Google Scholar]
- 4.Taylor KN, Eskander RN. PARP inhibitors in epithelial ovarian cancer. Recent Pat Anticancer Drug Discov. 2018;13:145–158. doi: 10.2174/1574892813666171204094822. [DOI] [PubMed] [Google Scholar]
- 5.Stecklein SR, Kumaraswamy E, Behbod F, Wang W, Chaguturu V, Harlan-Williams LM, Jensen RA. BRCA1 and HSP90 cooperate in homologous and non-homologous DNA double-strand-break repair and G2/M checkpoint activation. Proc Natl Acad Sci USA. 2012;109:13650–13655. doi: 10.1073/pnas.1203326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant HE, Helleday T. Inhibition of poly (ADP-ribose) polymerase activates ATM which is required for subsequent homologous recombination repair. Nucleic Acids Res. 2006;34:1685–1691. doi: 10.1093/nar/gkl108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, Giavara S, O'Connor MJ, Tutt AN, Zdzienicka MZ, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–8115. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 8.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 9.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 10.Kim G, Ison G, McKee AE, Zhang H, Tang S, Gwise T, Sridhara R, Lee E, Tzou A, Philip R, et al. FDA approval summary: Olaparib monotherapy in patients with deleterious germline BRCA-mutated advanced ovarian cancer treated with three or more lines of chemotherapy. Clin Cancer Res. 2015;21:4257–4261. doi: 10.1158/1078-0432.CCR-15-0887. [DOI] [PubMed] [Google Scholar]
- 11.Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, Korach J, Huzarski T, Poveda A, Pignata S, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 12.Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, Colombo N, Weberpals JI, Clamp A, Scambia G, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–1961. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 14.Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke GS, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen FC, van Overeem Hansen T, Sorensen CS. Hereditary breast and ovarian cancer: New genes in confined pathways. Nat Rev Cancer. 2016;16:599–612. doi: 10.1038/nrc.2016.72. [DOI] [PubMed] [Google Scholar]
- 16.Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, Roeb W, Agnew KJ, Stray SM, Wickramanayake A, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci USA. 2011;108:18032–18037. doi: 10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, Konecny GE, Coleman RL, Tinker AV, O'Malley DM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): An international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75–87. doi: 10.1016/S1470-2045(16)30559-9. [DOI] [PubMed] [Google Scholar]
- 18.Loibl S, O'Shaughnessy J, Untch M, Sikov WM, Rugo HS, McKee MD, Huober J, Golshan M, von Minckwitz G, Maag D, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomised, phase 3 trial. Lancet Oncol. 2018;19:497–509. doi: 10.1016/S1470-2045(18)30111-6. [DOI] [PubMed] [Google Scholar]
- 19.Bang YJ, Xu RH, Chin K, Lee KW, Park SH, Rha SY, Shen L, Qin S, Xu N, Im SA, et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1637–1651. doi: 10.1016/S1470-2045(17)30682-4. [DOI] [PubMed] [Google Scholar]
- 20.Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med. 2014;6:222ra18. doi: 10.1126/scitranslmed.3007154. [DOI] [PubMed] [Google Scholar]
- 21.Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA. 2005;102:13604–13609. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR, Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci USA. 2007;104:8749–8754. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, Khosh DB, Drisko J, Levine M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci USA. 2008;105:11105–11109. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du J, Martin SM, Levine M, Wagner BA, Buettner GR, Wang SH, Taghiyev AF, Du C, Knudson CM, Cullen JJ. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin Cancer Res. 2010;16:509–520. doi: 10.1158/1078-0432.CCR-09-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Wagner BA, Cramer-Morales KL, Furqan M, Sandhu S, Carlisle TL, Smith MC, Abu Hejleh T, et al. O2− and H2O2-mediated disruption of fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell. 2017;32:268. doi: 10.1016/j.ccell.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K, Rousseau C, Robitaille L, Miller WH., Jr Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol. 2008;19:1969–1974. doi: 10.1093/annonc/mdn377. [DOI] [PubMed] [Google Scholar]
- 27.Monti DA, Mitchell E, Bazzan AJ, Littman S, Zabrecky G, Yeo CJ, Pillai MV, Newberg AB, Deshmukh S, Levine M. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One. 2012;7:e29794. doi: 10.1371/journal.pone.0029794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welsh JL, Wagner BA, van't Erve TJ, Zehr PS, Berg DJ, Halfdanarson TR, Yee NS, Bodeker KL, Du J, Roberts LJ, II, et al. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): Results from a phase I clinical trial. Cancer Chemother Pharmacol. 2013;71:765–775. doi: 10.1007/s00280-013-2070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma E, Chen P, Wilkins HM, Wang T, Swerdlow RH, Chen Q. Pharmacologic ascorbate induces neuroblastoma cell death by hydrogen peroxide mediated DNA damage and reduction in cancer cell glycolysis. Free Radic Biol Med. 2017;113:36–47. doi: 10.1016/j.freeradbiomed.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stordal B, Timms K, Farrelly A, Gallagher D, Busschots S, Renaud M, Thery J, Williams D, Potter J, Tran T, et al. BRCA1/2 mutation analysis in 41 ovarian cell lines reveals only one functionally deleterious BRCA1 mutation. Mol Oncol. 2013;7:567–579. doi: 10.1016/j.molonc.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Wetering M, Barker N, Harkes IC, van der Heyden M, Dijk NJ, Hollestelle A, Klijn JG, Clevers H, Schutte M. Mutant E-cadherin breast cancer cells do not display constitutive Wnt signaling. Cancer Res. 2001;61:278–284. [PubMed] [Google Scholar]
- 32.Hall TA. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 33.Feldman AT, Wolfe D. Tissue processing and hematoxylin and eosin staining. Methods Mol Biol. 2014;1180:31–43. doi: 10.1007/978-1-4939-1050-2_3. [DOI] [PubMed] [Google Scholar]
- 34.Imai S, Kiyozuka Y, Maeda H, Noda T, Hosick HL. Establishment and characterization of a human ovarian serous cystadenocarcinoma cell line that produces the tumor markers CA-125 and tissue polypeptide antigen. Oncology. 1990;47:177–184. doi: 10.1159/000226813. [DOI] [PubMed] [Google Scholar]
- 35.Vaidyanathan A, Sawers L, Gannon AL, Chakravarty P, Scott AL, Bray SE, Ferguson MJ, Smith G. ABCB1 (MDR1) induction defines a common resistance mechanism in paclitaxel- and olaparib-resistant ovarian cancer cells. Br J Cancer. 2016;115:431–441. doi: 10.1038/bjc.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Food and Drug Administration, corp-author. FDA approves olaparib tablets for maintenance treatment in ovarian cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-tablets-maintenance-treatment-ovarian-cancer. [May 15;2018 ]; [Google Scholar]
- 37.Food and Drug Administration, corp-author. FDA approves rucaparib for maintenance treatment of recurrent ovarian, fallopian tube, or primary peritoneal cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-rucaparib-maintenance-treatment-recurrent-ovarian-fallopian-tube-or-primary-peritoneal. [May 15;2018 ]; [Google Scholar]
- 38.Ison G, Howie LJ, Amiri-Kordestani L, Zhang L, Tang S, Sridhara R, Pierre V, Charlab R, Ramamoorthy A, Song P, et al. FDA approval summary: Niraparib for the maintenance treatment of patients with recurrent ovarian cancer in response to platinum-based chemotherapy. Clin Cancer Res. 2018;24:4066–4071. doi: 10.1158/1078-0432.CCR-18-0042. [DOI] [PubMed] [Google Scholar]
- 39.Kamel D, Gray C, Walia JS, Kumar V. PARP inhibitor drugs in the treatment of breast, ovarian, prostate and pancreatic cancers: An update of clinical trials. Curr Drug Targets. 2018;19:21–37. doi: 10.2174/1389450118666170711151518. [DOI] [PubMed] [Google Scholar]
- 40.Polireddy K, Dong R, Reed G, Yu J, Chen P, Williamson S, Violet PC, Pessetto Z, Godwin AK, Fan F, et al. High dose parenteral ascorbate inhibited pancreatic cancer growth and metastasis: Mechanisms and a phase I/IIa study. Sci Rep. 2017;7:17188. doi: 10.1038/s41598-017-17568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panieri E, Santoro MM. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016;7:e2253. doi: 10.1038/cddis.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doskey CM, Buranasudja V, Wagner BA, Wilkes JG, Du J, Cullen JJ, Buettner GR. Tumor cells have decreased ability to metabolize H2O2: Implications for pharmacological ascorbate in cancer therapy. Redox Biol. 2016;10:274–284. doi: 10.1016/j.redox.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shibata A. Regulation of repair pathway choice at two-ended DNA double-strand breaks. Mutat Res. 2017;803-805:51–55. doi: 10.1016/j.mrfmmm.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 44.Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc Natl Acad Sci USA. 2011;108:3406–3411. doi: 10.1073/pnas.1013715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Do TV, Hirst J, Hyter S, Roby KF, Godwin AK. Aurora A kinase regulates non-homologous end-joining and poly(ADP-ribose) polymerase function in ovarian carcinoma cells. Oncotarget. 2017;8:50376–50392. doi: 10.18632/oncotarget.18970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott CL, Meier W, Shapira-Frommer R, Safra T, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–861. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 47.Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, Levine M. Vitamin C: Intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One. 2010;5:e11414. doi: 10.1371/journal.pone.0011414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ngo B, Van Riper JM, Cantley LC, Yun J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat Rev Cancer. 2019;19:271–282. doi: 10.1038/s41568-019-0135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.BRCA1 BRCA1 DNA repair associated [Homo sapiens (human)] https://www.ncbi.nlm.nih.gov/gene/?term=NM_007294. 2019 Nov 25; Gene ID: 672. Updated. [Google Scholar]
- 50.BRCA2 BRCA2 DNA repair associated [Homo sapiens (human)] https://www.ncbi.nlm.nih.gov/gene/?term=NM_000059. 2019 Nov 18; Gene ID: 675. Updated. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.