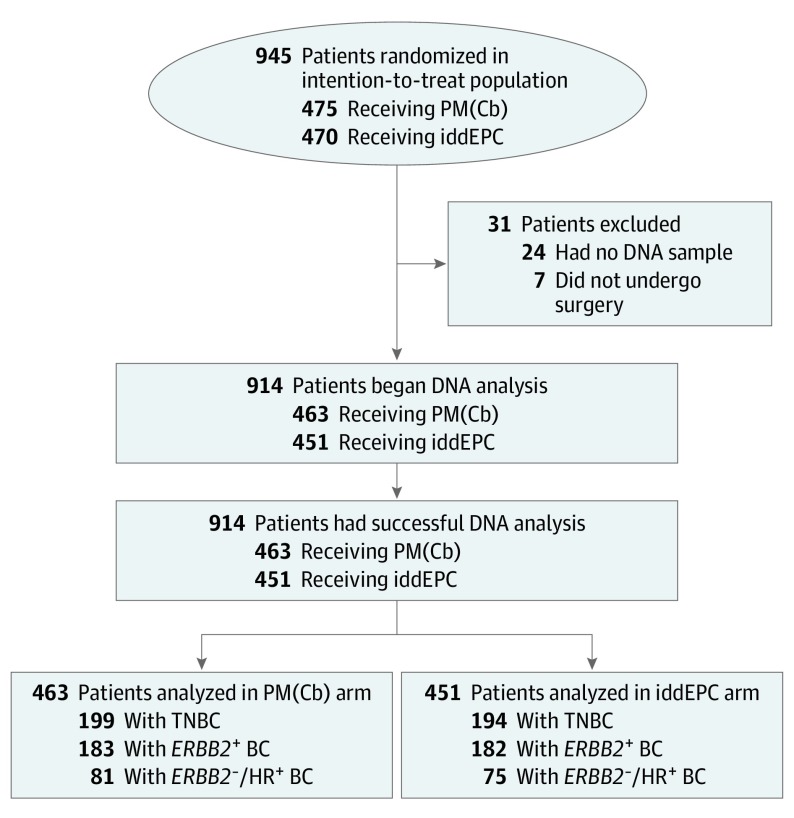
Figure 1. CONSORT Flow Diagram.
Between December 2014 and June 2016, 1204 patients were screened for eligibility, 961 patients were randomized at 57 sites, 945 started treatment (intention-to-treat population), and 938 underwent surgery.1 Of these 938 patients, 24 patients were not included in this secondary analysis because no DNA sample was available. BC indicates breast cancer; CONSORT, Consolidated Standards of Reporting Trials; HR, hormone receptor; iddEPC, intense dose-dense epirubicin, paclitaxel, and cyclophosphamide; PM(Cb), paclitaxel and nonpegylated liposomal doxorubicin with or without carboplatin; TNBC, triple-negative BC.