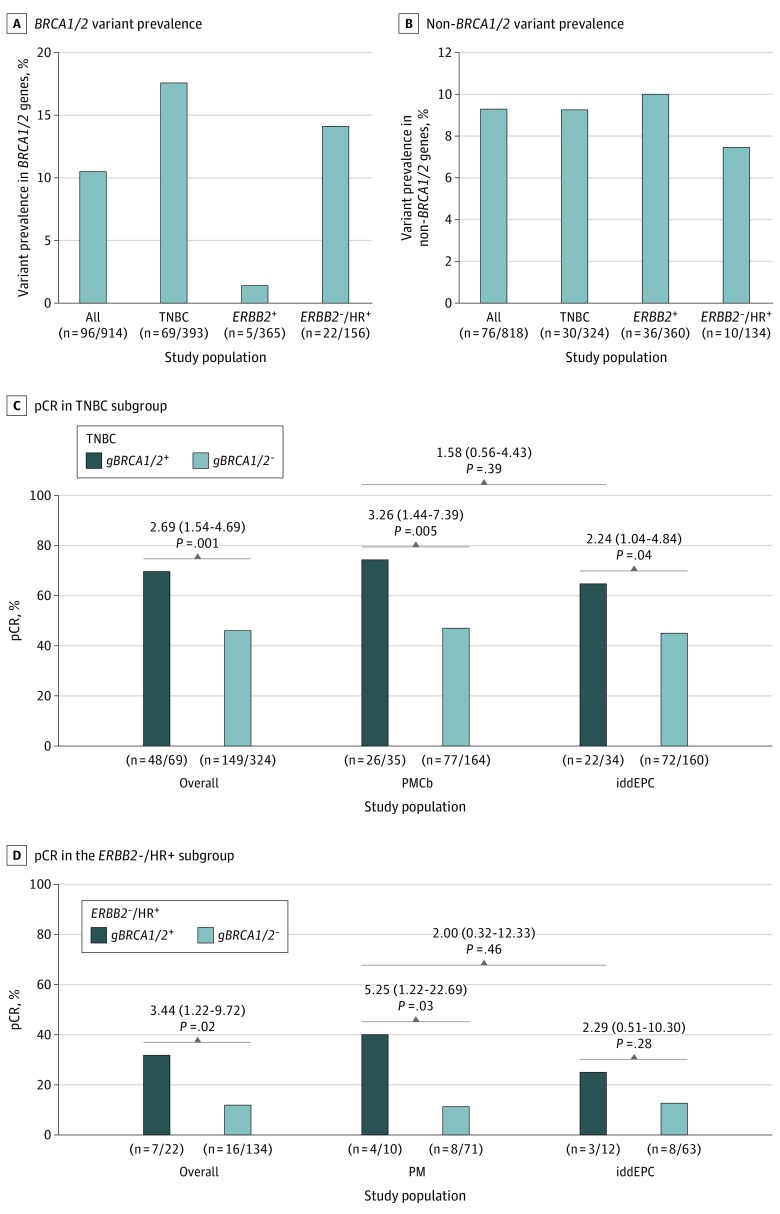
Figure 2. Germline Variant Prevalence and Pathologic Complete Response (pCR) Rates According to Variant Status and Study Arm.
A, Number of patients with germline BRCA1/2 (gBRCA1/2) variants in the overall study sample (n = 914) and according to biological subtype (triple-negative breast cancer [TNBC]; ERBB2-positive breast cancer; ERBB2-negative, hormone receptor [HR]–positive breast cancer). B, Number of patients with germline variants in non-BRCA1/2 genes among the subgroup of patients without gBRCA1/2 variants (n = 818) overall and according to the biological subtype. C and D, Rates of pCR according to gBRCA1/2 variant status in the TNBC subgroup (C) and in the ERBB2-negative, HR-positive subgroup (D), overall and by study arm. For the comparison of pCR rates according to gBRCA1/2 variant status, odds ratios (95% CI) are shown (univariate logistic regression). Abbreviations: iddEPC, intense dose-dense epirubicin, paclitaxel, and cyclophosphamide; PM, paclitaxel and nonpegylated liposomal doxorubicin; PMCb, PM with carboplatin.