Figure 1.
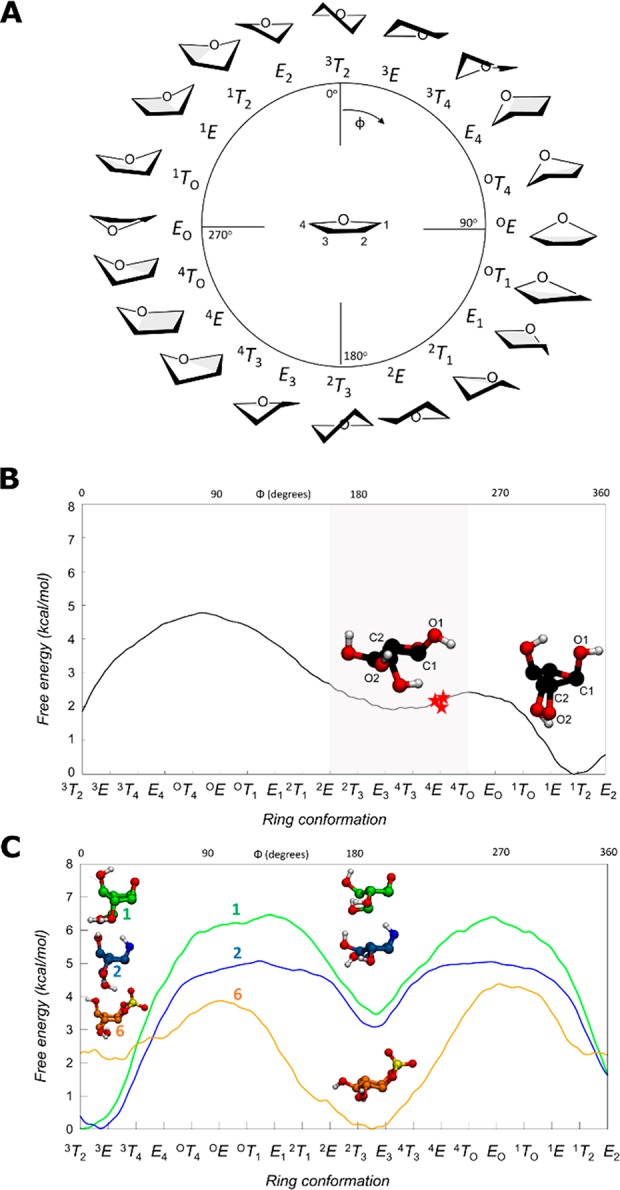
(A) Graphical representation of the conformations of a 5-membered ring according to the Cremer–Pople angle ϕ. (B) Conformational FEL of isolated α-l-arabinofuranose. Conformations observed in Michaelis complexes of α-l-arabinofuranosidases are represented with a red star (PDB 2VRQ and 1QW9 for GH51 and PDB 6SXR, this work, for GH54). The conformational region having an equatorial O2 is shaded. (C) Conformational FEL of α-l-arabinofuranose-configured cyclophellitol (1), aziridine (2), and cyclic sulfate (6).
