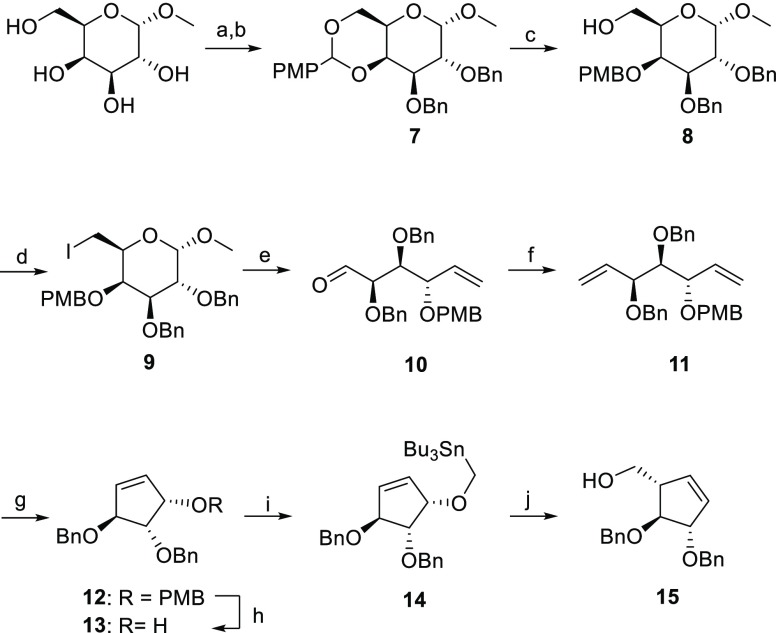
Scheme 1. Synthesis of l-Arabinofuranose-Configured Cyclopentene 15.
Reagents and conditions: (a) (1S)-(+)-10-camphorsulfonic acid, CH3CN, 50 °C, 300 mbar, 2.5 h; (b) BnBr, NaH, TBAI, DMF, 0 °C, rt, 18 h, 74% over two steps; (c) BH3·THF, Bu2BOTf, DMF, 0 °C, 15 min, 90%; (d) I2, TPP, THF, reflux, 3 h, 79%; (e) activated Zn powder, THF, 35 °C, 2 h, 84%; (f) Ph3PCH3Br, n-BuLi, THF, −78 to −20 °C for 1 h, then rt, 18 h, 73%; (g) Grubb’s II cat., DCM, reflux, 18 h, 90%; (h) DDQ, DCM, 0 °C, rt, 2 h, 86%; (i) Bu3SnMeI, KH, dibenzo-18-crown-6, THF, 0 °C, rt, 18 h, 91%; (j) n-BuLi, THF, −78 °C to rt, 18 h, 68%.