Abstract
Objective:
Smoking is generally accepted as a factor that affects the disease course in inflammatory bowel disease patients. Whether these effects can be contributed to the immunomodulatory effects of nicotine via nicotinic acetylcholine receptor (nAChR) activation is unclear. As previous data suggest that the α7 nicotinic acetylcholine receptor (CHRNA7) and its duplicated variant CHRFAM7A may specifically participate in the inflammatory response of monocytes, we evaluated whether repeated nicotine exposure or smoking affects monocyte CHRNA7 and CHRFAM7A expression and cholinergic immunomodulation.
Methods:
The human monocyte cell line THP-I was incubated with nicotine for different time points before endotoxin exposure. In a pilot volunteer study using smoking (n = 4) and nonsmoking (n = 7) individuals, vagal output was stimulated by olive oil administration after which monocytes were analyzed for nicotinic receptor expression. Serum tumor necrosis factor (TNF) levels were determined using ELISA and expression levels of the nAChR subunits CHRNA7, CHRNB2 or CHRFAM7A were analyzed using QPCR. Results: Repeated nicotine exposure upregulated CHRNA7 expression on THP-I monocytes and led to an enhanced potential of α7 nAChR agonist GSK1345038A to reduce TNF levels. Furthermore, CHRNA7 was only detectable in isolated blood monocytes of smokers. On the other hand, the expression of CHRFAM7A and CHRNB2 was not affected by nicotine exposure. Lipopolysaccharides-induced TNF secretion was inhibited by nicotinic receptor activation in THP-I monocytes, but this response was not consistently seen in blood monocytes from smoking individuals.
Conclusions:
We conclude that CHRNA7 expression on blood monocytes is upregulated in smoking individuals, which may contribute to cholinergic immunomodulation.
Key Words: Acetylcholine, Monocytes, Macrophages, Alpha7 nicotinic receptor, Tumor necrosis factor
Introduction
It is increasingly recognized that the vagus nerve has immunomodulating properties via activation of cholinergic receptors on nonneuronal cells. Cholinergic signaling attenuates proinflammatory cytokine release and improves clinical outcome in experimental animal models of inflammatory disease, such as sepsis [1], pancreatitis [2], postoperative ileus [3] and rheumatoid arthritis [4,5]. It has been proposed that electric stimulation of the efferent vagus nerve leads to the release of acetylcholine (ACh), which may activate nicotinic acetylcholine receptors (nAChRs) that are broadly expressed on different types of immune cells [6,7]. Activation of the nAChRs has been shown to result in the attenuation of proinflammatory cytokine release in monocytes and macrophages in vitro[8]. However, more recent results indicate that the vagal immunomodulation of systemic inflammatory responses involves activation of adrenergic splenic nerve fibers [9].
Earlier studies have indicated that the anti-inflammatory effect of ACh is mediated through the α7 nicotinic acetylcholine receptor (CHRNA7) expressed on the surface of primary human monocytes [10], while other nAChR subtypes such as CHRNB2 can affect cellular macrophage functions such as phagocytosis [8]. Besides the CHRNA7, a partially duplicated hybrid form of this gene was identified (CHRFAM7A) in which exon 5–10 of the α7 genes have been duplicated in a ‘tail to head’ organization and combined with four novel exons (A–D) to comprise a new gene [11]. The CHRFAM7A gene transcript is expressed in human monocytes and has been identified in brain and human monocyte cell lines [12]. This CHRFAM7A mRNA is transcribed to a 45-kDa protein which lacks a nicotine binding domain, and hence it is unclear whether it is part of a functional nicotinic receptor [13]. However, recent work indicates that its expression is transcriptionally regulated by lipopolysaccharides and cytokines such as IL-1β [14], and may contribute to the cholinergic regulation of the immune response [12]. Furthermore, recent studies have demonstrated that the protein encoded by CHRFAM7A is a dominant negative regulator of the number of functional α7 nAChRs on the surface [14].
Importantly, as most of the above work considering the cholinergic inhibition of inflammation has been done in vitro and in experimental mouse models, the relevance of cholinergic immunomodulation to human disease remains sketchy. In inflammatory bowel diseases such as Crohn's disease and ulcerative colitis, cholinergic immunomodulation may play a significant role providing therapeutic opportunities for its treatment. Ulcerative colitis is characterized by an excessive innate immune response to commensal flora, and it is widely acknowledged that smoking has a protective effect in the development of ulcerative colitis and reduces its severity [15,16,17,18,19]. Smoking affects nicotinic receptor expression in rodent models [20] and nicotine affects the course of experimental colitis in mouse models [21,22]. Based on clinical and experimental observations, nicotine patches and nicotine enemas have been tested in ulcerative colitis patients, with variable outcomes as compared to standard therapy [23]. Hence, the protective mechanism and its clinical implications remain unknown but may implicate nicotinic receptor activation.
In the current study we reasoned that the beneficial effect of smoking in ulcerative colitis patients may be explained by the fact that the repeated exposure to nicotine may lead to sensitization to the ‘cholinergic anti-inflammatory pathway’. Human immune cells express a variety of nAChRs, also subject to genetic or environmental factors [7,24,25]. One of these environmental factors resulting in altered nAChR expression could be cigarette smoking. In brain regions, it has already been demonstrated that long-term consumption of tobacco elicits nAChR upregulation [18,26], although it is unclear whether this upregulation coincides with enhanced protein expression. Hence, in this study, we hypothesized that regular exposure to nicotine in tobacco smoke affects CHRNA7 and CHRFAM7A expression on human monocytes. The aims of the study were to determine whether priming to nicotine, either by cigarette smoking or by in vitro nicotine exposure, altered nAChR expression levels on human monocytes. Moreover, we determined whether repeated nicotine exposure rendered human peripheral blood monocytes more susceptible to cholinergic immunomodulation by testing the effect of enhanced vagal output using high-fat (olive oil) intake as reported earlier [27,28,29].
We show here that repeated exposure with nicotine or tobacco smoking specifically elevates nAChR CHRNA7 expression on human monocytes. This coincided with an enhanced sensitivity to α7 nAChR agonists. However, this nicotine priming or cigarette smoking does not consistently affect LPS-induced tumor necrosis factor (TNF) production in human monocytes or whole blood samples. In addition, our data indicate that human peripheral blood monocytes show a large intraindividual variation in response to nAChR activation or high-fat nutrition in vivo, which is shown to activate the cholinergic anti-inflammatory pathway [29,30].
Materials and Methods
Cell Culture and Cell Treatment
The human acute monocytic leukemia cell line THP-I was cultured in RPMI 1640 (Sigma Aldrich), supplemented with 1% fetal bovine serum, 100 units/ml penicillin, 100 µg/ml streptomycin and 2 mML-glutamine. Cells were maintained at a recommended density between 2 × 105/ml and 1 × 106/ml at 37°C in 5% CO2. Before the experiments, cells were seeded at a density of 1 × 106/ml in 12-well plates. Cells were incubated with different doses of nicotine (0, 1, 10 µM) at the time points indicated, followed by vehicle or 10 ng/ml LPS challenge for 2 h. GSK1345038 (supplied by GlaxoSmithKline, Stevenage, UK) was added to the cell culture in a medium at the indicated concentrations for 20 min prior to the LPS challenge. The medium was subsequently collected and TNF levels were measured using sandwich ELISA (R&D Systems, Minneapolis, Minn., USA).
RNA Isolation and Polymerase Chain Reaction
For RNA isolation, 1 × 106 THP-I or 2 × 106 whole-blood isolated monocytes were centrifuged (8 min, 1,500 rpm), washed in ice-cold PBS and the total RNA was extracted using the RNeasy minikit (Qiagen, Venlo, The Netherlands) according to the provided protocol. The RNA concentration was determined using a nanodrop ND-1000 spectrophotometer (Thermo scientific). Subsequently, samples were treated with DNase and reverse transcribed. Amplification from 100 ng of RNA was carried out using Reddymix (Thermoscientific) in a thermocycler with 30 cycles of denaturation at 94°C for 30 s, annealing at 60° for 30 s and elongation for 30 s at 72°C. The bands were visualized on an ethidium bromide-stained 2.5% agarose gel. For LightCycler analysis, the complementary DNA (cDNA; 100 ng) was subjected to a LightCycler polymerase chain reaction (Roche, Woerden, The Netherlands) for 35 cycles at 60°C. The following primers were used: CHRNA7, forward: 5′-CCCAAGTGGACCAGAGTCAT-3′, reverse: 5′-GCCACACACTACCCCAGAGT-3′; CHRFAM7A, forward: 5′-GGAGGTGAGGGGAAGATGTC-3′, reverse: 5′-CAGGTCCTGCTGACTCAGGT-3′; CHRNB2, forward: 5′-TGGGAAGATTATCGCCTCAC-3′, reverse: 5′-AGACCACGGCATTGGAATAG-3′. B2M was used as a reference gene.
Volunteers' Olive Oil Study
Eleven healthy volunteers (6 male, 5 female; aged 25–35) were recruited. All volunteers refrained from caffeine/alcohol-containing beverages, smoking and food for at least 12 h before blood collection. At 8.30 a.m., 60 ml of venous blood was drawn from the cubital vein into sodium heparin tubes (Vacutainer system, BD Biosciences, Plymouth, UK). Fifteen minutes afterwards, 50 ml (44 g) of olive oil (extra-virgin) was orally administered to the volunteers in a single dose. Thirty minutes after intake, another 60 ml of venous blood was drawn. Characteristics of the 11 healthy subjects considering gender, age and smoking habits are represented in table 1. The smokers included were all regular daily smokers.
Table 1.
Characteristics of the study subjects
| Subject No. | Gender | Age | Smoking status |
|---|---|---|---|
| 1 | F | 29 | + |
| 2 | F | 30 | + |
| 3 | M | 29 | + |
| 4 | M | 33 | + |
| 5 | F | 22 | − |
| 6 | M | 27 | − |
| 7 | M | 26 | − |
| 8 | F | 25 | − |
| 9 | M | 29 | − |
| 10 | F | 31 | − |
| 11 | M | 30 | − |
Whole Blood Assay
Whole blood was diluted 1:1 in RPMI 1640 medium supplemented with antibiotics [100 U/ml penicillin and 100 µg/ml streptomycin (Sigma-Aldrich, Mannheim, Germany)] and L-glutamine. The blood was incubated in 96-well plates (total volume 200 µl/well) and pretreated with nicotine (0–1,000 nM) or vehicle. Thirty minutes after nicotine pretreatment, the whole blood samples were stimulated with 10 ng/ml LPS for 4 h. TNF levels were determined using sandwich ELISA.
Monocyte Isolation
For the isolation of monocytes of the 11 healthy volunteers, 50 ml of anticoagulated blood was diluted with buffered saline (PBS). Aliquots (25 ml) of this suspension were layered over an equal volume of Ficoll-Hypaque (GE Healthcare, Little Chalfont, UK) and gradients were subjected to centrifugation (800 g for 20 min at room temperature). Peripheral blood mononuclear cells (PBMCs) were washed three times in sterile PBS, after which they were counted. CD14+ cells were positively separated by high-gradient magnetic sorting using the MIDI-magnetic cell sorting (MACS) and Clini-MACS techniques according to the protocol provided by the manufacturer (Miltenyi Biotec, Bergisch Gladbach, Germany). In short, PBMCs were resuspended in MACS buffer (0.5% bovine serum albumin, 2 mM EDTA in PBS) at a density of 12.5 × 107 cells/ml, CD14 MicroBeads (20 µl/107 cells) were added and the samples were kept at 4°C for 15 min. Subsequently, cells were washed in 10 ml buffer by centrifugation (8 min, 300 g, 4°C), resuspended in buffer (500 µl/108 cells) and transferred to LS columns placed in the magnetic field of the MACS separator. CD14+ cells were selected by adhesion to the magnetized column, supernatant-containing unselected cells were discarded and the labeled and positively enriched cells were eluted after removal of the columns from the magnetic device.
Statistical Analysis
Statistical analysis was performed in Prism (GraphPad) version 4. The Friedman 2-way ANOVA was used to explore multiple dependent value assays. If the Friedman analysis was significant, individual values compared with the saline were tested with a Mann-Whitney U test. p values <0.05 were considered statistically significant, and results were expressed as mean ± SEM.
Results
Nicotine Significantly Reduces TNF Production in Human Monocytes
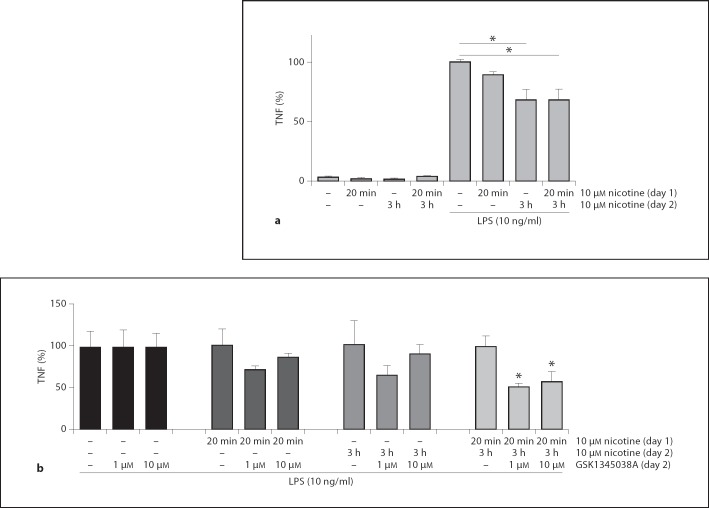
To determine whether nicotine reduced TNF production in human monocytes, we preincubated the human monocyte THP-I cell line with different doses of nicotine prior to LPS challenge (10 ng/ml). In line with previous data [31], nicotine caused a concentration-dependent inhibition of the LPS-induced TNF production in THP-1 cells. At the highest concentration of 10 µM nicotine, TNF production was significantly reduced from 100 ± 5.4% down to 68.3 ± 3.4% of vehicle (fig. 1).
Fig. 1.
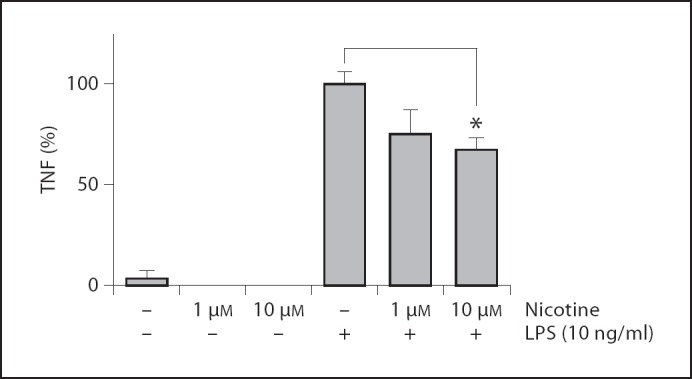
Nicotine attenuates TNF production in human THP-I monocytes. THP-I cells were pretreated with 0, 1 or 10 µM of nicotine for 30 min, before 0 or 10 ng/ml LPS challenge. After 3 h, TNF levels were measured using ELISA. Data are mean ± SEM of two independent experiments done in triplicate. * p < 0.05.
Nicotine Priming Induces CHRNA7 Expression in Human Monocytes
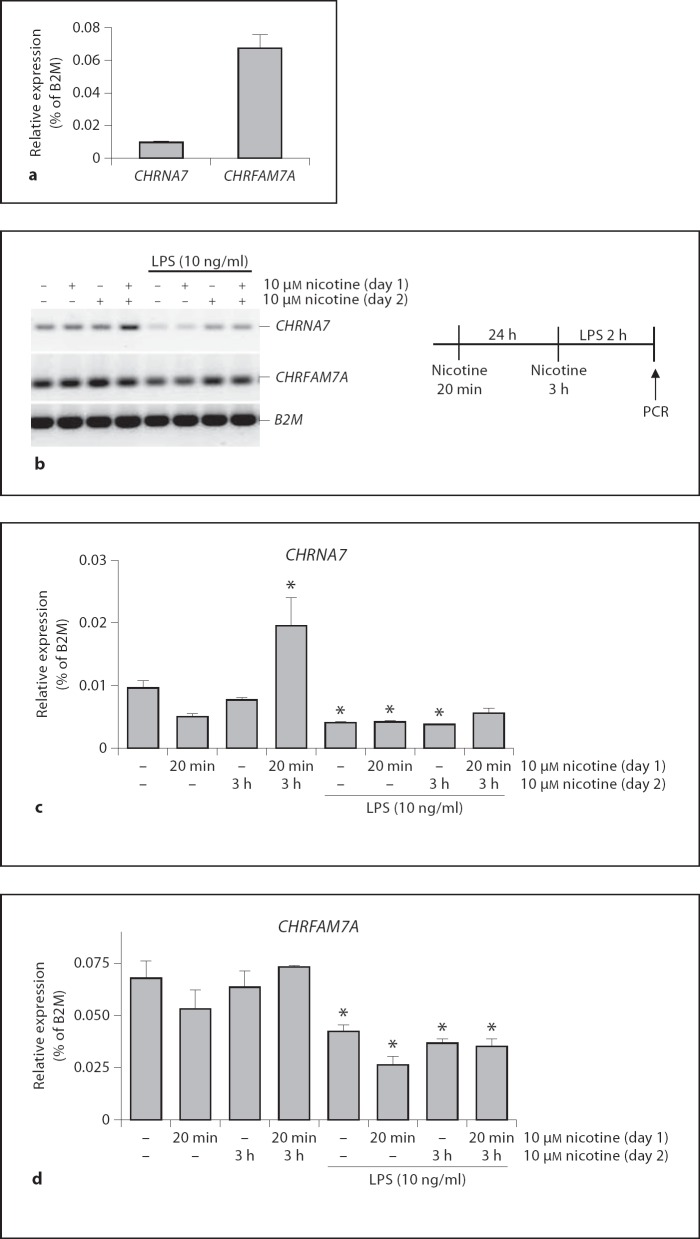
Next, we evaluated the expression of nAChRs that are involved in immunoregulation. As is shown in figure 2a, THP-I cells express both CHRNA7 and CHRFAM7A although the expression of CHRFAM7A is 7-fold higher than the CHRNA7 expression, confirming earlier reports [14]. CHRNA7 expression levels are low, but not undetectable, as was reported earlier [12]. To relate the CHRNA7 transcriptional expression pattern to repeated nicotine exposure in smokers' monocytes, we next tested whether repeated exposure to nicotine-induced CHRNA7 expression on human immune cells. To this end, we primed human THP-I monocytes with vehicle or nicotine (10 µM) for 20 min. After 24 h, cells were reexposed to nicotine or vehicle for another 3 h and subsequently they were challenged with LPS. As is shown in figure 2b, nicotine priming at day 1, followed by 3 h of nicotine reexposure at day 2, induced CHRNA7 expression in THP-I cells. Short (20 min) preincubation with nicotine, or single 3-hour exposure, did not alter CHRNA7 expression levels in monocytes (fig. 2c). However, when cells were primed with nicotine and reexposed to nicotine 24 h later, expression levels of CHRNA7 were significantly enhanced (fig. 2c). As the CHRNA7 has been associated with inflammatory responses, we next examined whether expression of CHRNA7 might be modified by proinflammatory stimuli. In figure 2c it is shown that in LPS-stimulated THP-I monocytes, CHRNA7 expression was significantly downregulated as compared to non-LPS-challenged cells. When cells were primed and reexposed to nicotine before endotoxin challenge, this downregulation was no longer significant. This indicates that repeated nicotine exposure also induces CHRNA7 expression in monocytes upon TLR activation. In addition to CHRNA7 expression, we also determined the effects of nicotine exposure on expression levels of the CHRFAM7A. Nicotine priming or reexposure to nicotine did not alter CHRFAM7A expression levels, as is shown in figure 2b and d. In line with earlier studies [12,14], the levels of the CHRFAM7A transcript were significantly downregulated in the LPS-treated cells to about 60% of the untreated cells.
Fig. 2.
CHRNA7 and CHRFAM7A expression in THP-I cells after nicotine exposure. a mRNA of THP-I cells was isolated and expression levels of CHRNA7 and CHRFAM7A were determined by QPCR. B2M was used as a reference gene. b THP-I cells were primed with vehicle or 10 µM nicotine at day 1 for 20 min and washed afterwards. 24 h later, at day 2, cells were treated with vehicle or nicotine for 3 h, washed and challenged with 0 or 10 ng/ml LPS for 2 h. A scheme of the experimental set-up is represented in the right panel. RNA was isolated and CHRNA7 and CHRFAM7A expression levels were determined using RT-PCR. PCR products were visualized on agarose gel electrophoresis. CHRNA7 (c) and CHRFAM7A (d) mRNA levels were expressed as a percentage of B2M expression. Data are representative of three independent experiments in duplicate. * p < 0.05, differences from vehicle-treated and unstimulated cells using Mann-Whitney U test.
Repeated Nicotine Exposure Leads to an Enhanced Expression of a Functional α7 nAChR
Next, we determined the effect of nicotine priming, followed by nicotine reexposure on cytokine production in human THP-I monocytes. In figure 3a, it is shown that nicotine exposure for 3 h reduces LPS-induced TNF production from 100 ± 2.3 to 68 ± 7.9%. Nicotine priming for 20 min followed by nicotine reexposure attenuated LPS-induced TNF production in THP-I cells although it did not lead to further attenuation of the TNF response if compared to a single nicotine exposure. Given the observation that nicotine priming and reexposure enhanced CHRNA7 transcript expression, we next assessed whether a selective α7nAChR agonist was effective in attenuating TNF release from LPS-challenged cells that were primed with nicotine. Indeed, repeated nicotine treatment (priming and reexposure; fig. 3a, b) significantly reduced TNF release by α7 nAChR agonist GSK1345038 (fig. 3b), indicating that the enhanced CHRNA7 transcription after nicotine priming led to a functionally active protein.
Fig. 3.
Repeated nicotine exposure leads to an enhanced expression of a functional α7 nAChR. a Human THP-I monocytes were preincubated for 20 min with 1 µM nicotine or vehicle at day 1. At day 2 (24 h later), cells were reexposed with 10 µM nicotine for 3 h, followed by 2 h of saline or 10 ng/ml LPS stimulation. b TNF levels of THP- 1 monocytes stimulated with LPS and nicotine at the indicated dosing scheme. Cells were subsequently incubated with GSK1345038 nAChR α7-specific agonist at the indicated doses. Values are given as percentages relative to 0 µM GSK1345038 incubation for each treatment group. Data shown are mean ± SEM of three independent experiments done in duplicate. * p < 0.05.
CHRNA7 Is Present on Monocytes of Cigarette Smokers, but Not on Monocytes of Nonsmoking Individuals
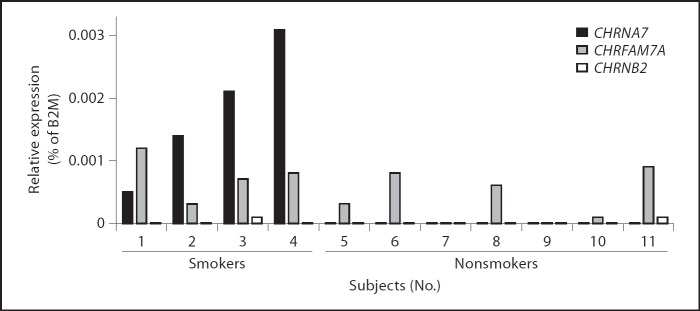
Since nicotine priming enhanced CHRNA7 expression in human THP-I cells, we questioned whether CHRNA7 expression levels are elevated in human monocytes of regular cigarette smokers as compared to nonsmokers. To address this, we collected whole blood samples from 11 healthy volunteers. The characteristics considering gender, age and smoking habits are represented in table 1. We isolated monocytes from these whole blood samples and quantified the RNA levels of CHRNA7, CHRFAM7A and CHRNB2, i.e. nicotinic receptors that were previously implicated in the anti-inflammatory potential of nicotine. CHRNA7 expression was only observed in monocytes of smoking individuals (fig. 4, subjects 1–4), while the other 7 subjects lacked expression of this receptor. On the other hand, CHRFAM7A was detected on monocytes of 10 individuals, whereas CHRNB2 was expressed on monocytes of 2 volunteers (fig. 4, subjects 3 and 11). These data indicate that, among the nAChRs analyzed, cigarette smoking is associated with expression of CHRNA7 on circulating monocytes.
Fig. 4.
nAChR expression levels on circulating monocytes isolated from whole blood samples from 11 healthy volunteers. Subjects 1–4 are regular cigarette smokers, while the other 7 are nonsmokers. The presence of CHRNA7, CHRFAM7A and CHRNB2 mRNA was determined using QPCR, presented as a percentage of B2M expression.
Nicotinic Potential to Reduce Whole Blood TNF Release Varies per Individual
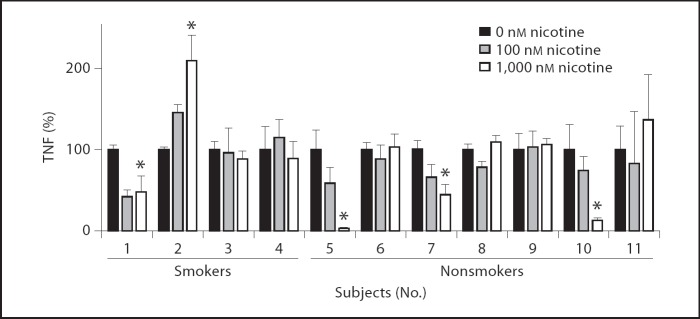
Todetermine the functional effect of nicotine on LPS-induced TNF production in whole blood samples of the healthy volunteers, we preincubated whole blood samples for 30 min with different concentrations of nicotine, followed by 4 h LPS stimulation. In line with the THP-1 monocytes, nicotine significantly reduced TNF production in the whole blood samples of 4 healthy subjects, varying from approximately 56–97% reduction in TNF release at a concentration of 1,000 nM nicotine (fig. 5, subjects 1, 5 and 10). Notably, higher concentrations of nicotine as used for THP-1 cells did not lead to further decrease of TNF response. However, in the whole blood samples of 7 healthy subjects, LPS-induced TNF levels were unaffected by nicotine, whereas 1 person showed a significant increase in TNF release (210 ± 31% increase; fig. 5, subject 2). Remarkably, in the whole blood samples of the 4 smokers (subjects 1–4), nicotine reduced LPS-induced TNF production in only 1 person, was ineffective in 2 others and enhanced TNF production in another. These data indicate that, despite the finding that cigarette smoking induced the expression of CHRNA7 on monocytes, it did not render whole blood samples more susceptible to nicotinic reduction of TNF.
Fig. 5.
Nicotinic effects on LPS-induced TNF production in whole blood samples of healthy volunteers. Whole blood samples of healthy volunteers (subjects 1–4 were smokers) were incubated with nicotine (0–1,000 nM at the concentrations indicated) for 30 min, followed by 4 h LPS stimulation (10 ng/ml). TNF levels in the medium were measured using ELISA and represented as a percentage of vehicle treatment. The experiment was performed in quadruple. * p < 0.05.
TNF Secretion after Olive Oil Intake in Smokers and Nonsmokers
Given the enhanced CHRNA7 expression in blood monocytes derived from smoking individuals, we next studied whether smoking would sensitize these cells to cholinergic immunomodulation in vivo. As high-fat enteral nutrition was demonstrated to be an effective stimulant for the cholinergic anti-inflammatory pathway [28,30], we tested the effect of oral ingestion of long-chain fatty acids in the form of olive oil on the TNF production by monocytes. A schematic overview of the study design is represented in figure 6.
Fig. 6.
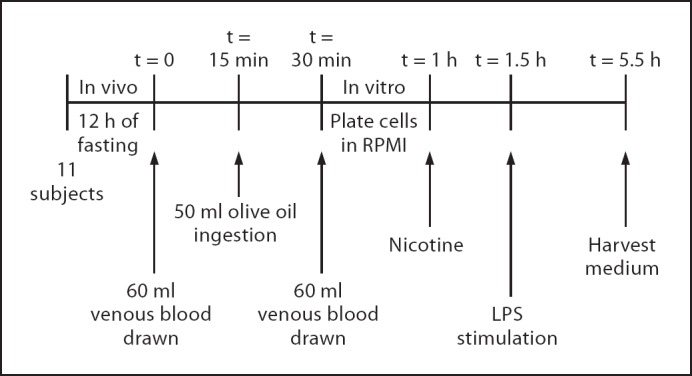
Design of the olive oil study. Eleven healthy volunteers participated in the study, they refrained from food and caffeine/alcohol-containing beverages for 12 h. At t = 0, 60 ml of venous whole blood was taken, followed by 50-ml oral ingestion of extra-virgin olive oil at t = 15 min. Another 60 ml of whole blood was drawn at t = 30 min. Whole blood was diluted 1:1 with RPMI medium, cells were plated and preincubated with 10 µM nicotine at t = 1 h, followed by 10 ng/ml LPS stimulation at t = 1.5 h. After 4 h, the medium was harvested and TNF levels were measured using ELISA.
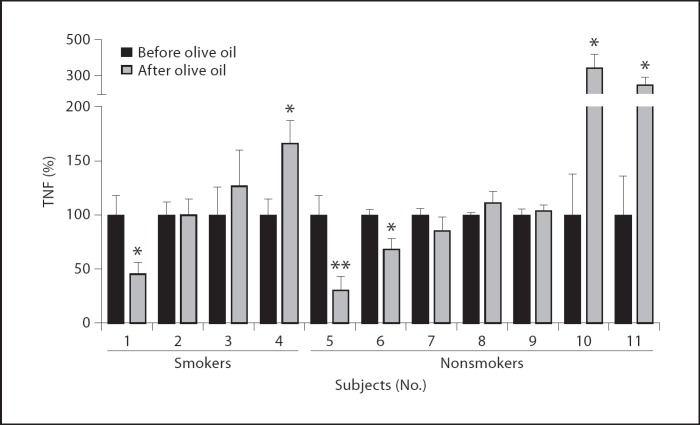
In 3 healthy subjects, LPS-induced TNF production was significantly diminished after olive oil intake, with a maximum inhibition of 50.4 ± 7.7% (fig. 7, subjects 1, 5 and 6). On the other hand, in 3 volunteers, oral administration of olive oil enhanced endotoxin-challenged TNF release up to 249 ± 46% (fig. 7, subjects 4, 10 and 11), whereas olive oil administration had no effect on whole blood stimulation in the other 5 subjects. No difference was observed between olive oil effects on the whole blood samples of smoking subjects (No.1–4) as compared to nonsmokers (No. 5–11).
Fig. 7.
Effects of olive oil administration on LPS-induced TNF production in whole blood. Whole blood samples were administered before and 30 min after 50 ml of oral olive oil administration, challenged with 10 ng/ml LPS for 4 h. TNF levels were determined and depicted as a percentage of TNF secretion before olive oil administration. Data are represented as mean ± SEM of one experiment done 8-fold. * p < 0.05; ** p < 0.01.
Discussion
The finding that nicotine inhibits the activation of immune cells, together with the observation that vagus nerve signaling or specific CHRNA7 agonists attenuate disease in several inflammatory animal models [29], implies that therapeutic agents modifying cholinergic signaling might be beneficial in humans. However, since vagus nerve stimulation in humans is a rather invasive procedure, data on human studies are limited.
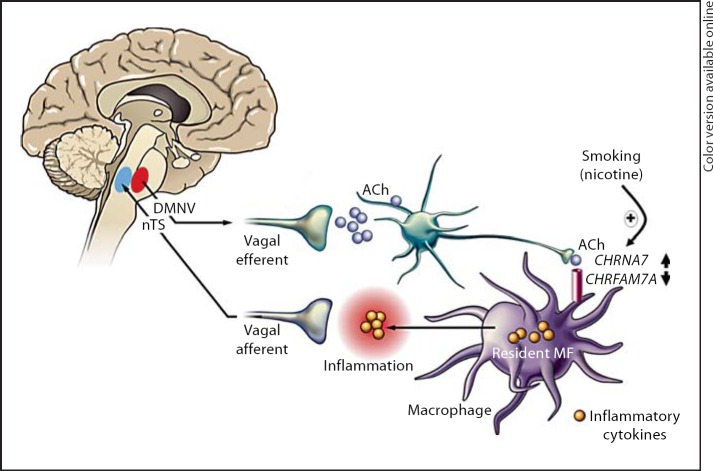
Cigarette smoking is an important environmental factor in ulcerative colitis. Ulcerative colitis patients with a history of smoking usually acquire their disease after they have stopped smoking [17,18,19]. Patients who smoke intermittently often experience an improvement in their colitis symptoms during the periods when they smoke [15,16,18]. The mechanism behind the protective effect of smoking in ulcerative colitis remains obscure. The effects of smoking and nicotine are numerous and since the pathogenesis of inflammatory bowel disease is only partially understood, any discussion on the possible mechanisms can only be speculative. Potentially, nicotine in cigarette smoke can restrain the immune response in ulcerative colitis via activation of nAChRs on immune cells, which may explain its protective effect. Given the purported role of CHRNA7 in mediating the cholinergic anti-inflammatory pathway [10], we hypothesized that chronic nicotine exposure affects CHRFAM7A and CHRNA7 expression on human monocytes, thereby sensitizing these cells to the cholinergic anti-inflammatory pathway (see scheme in fig. 8).
Fig. 8.
Scheme of the proposed mechanism. Nicotine upregulates alpha7 nAChR expression on monocytes from smokers, sensitizing monocytes to the anti-inflammatory actions of nicotine. Modified according to van der Zanden et al., Neurogastro and Mot, 2009.
In this study, we have demonstrated that repeated nicotine exposure upregulated CHRNA7 expression on THP-I monocytes. In conjunction, in a volunteer study, CHRNA7 was only detectable on monocytes of smoking individuals, while it was lacking on monocytes of nonsmokers. Despite the limited group size, these data suggest that enhanced CHRNA7 monocyte expression by repeated nicotine exposure could be a factor that has not been fully explored in the protective effect of smoking in ulcerative colitis. However, enhanced CHRNA7 expression did not render human whole blood monocytes more susceptible to nicotinic immunomodulation, which may indicate that the CHRNA7 is not the only mediator effectuating the cholinergic anti-inflammatory effect and another nAChR might be involved. For example, the finding that CHRFAM7A is downregulated in monocytes by LPS challenge, and has been found to be downregulated in smokers' PBMCs [32] suggests that it could play a role in inflammation by affecting functional α7 nAChRs [14]. This is in conjunction with earlier studies in which it was put forward that receptors of mixed subunits of CHRNA7 and CHRFAM7A are formed that can modulate the signaling potential of immune cells to respond to ACh released from the vagus nerve during infection [12].
In human subjects, we failed to demonstrate a direct relation between CHRNA7 expression and TNF release. In this respect, it should be noted that alternative mechanisms may play a role in vagal immunomodulation in vivo. Recent data demonstrate, for instance, a crucial role of the spleen in relaying the anti-inflammatory effects of vagus nerve activity, as electrical vagus nerve stimulation fails to attenuate serum TNF levels in splenectomized mice treated with endotoxin [33]. It was concluded that ACh released by the vagus nerve does not reach the spleen directly, but acts on CHRNA7 at the level of the ganglia of the celiac-superior mesenteric plexus to modulate splenic nerve function. In line with this it was recently shown that attenuation of TNF production by spleen macrophages induced by vagus nerve stimulation is mediated by norepinephrine released from splenic nerve endings [34], which may stimulate specific T cell populations to secrete ACh [9]. These data put the role of the CHRNA7 in a new perspective; although in vitro ACh modulates macrophage function via CHRNA7, the in vivo effects of vagus nerve stimulation may rely on neuronal CHRNA7, rather than on monocytes or macrophages. This implies that, although nAChR expression on circulating monocytes in humans is rather low, cholinergic activation could still display in vivo immunomodulating functions via neuronal nAChRs [34], or other cell lymphoid cell types such as T cells [9].
We tested the effect of nicotine pretreatment on TNF production by taking endotoxin-stimulated whole blood samples of 11 healthy volunteers. We chose whole blood samples since this was the closest to the in vivo situation and cytokines produced from whole blood were found to be strongly correlated with monocytic cytokines [35]. Nicotine dose dependently reduced TNF production in 4 out of 11 human whole blood samples, whereas in one sample TNF release was enhanced. Other studies report that in other cell types, such as peripheral blood-derived human macrophages [1] and mononuclear cells [36], nicotinic receptor activation reduced TNF production in all samples. On the other hand, Kox et al. [37] reported that nicotine attenuated TNF release equally on PBMCs, monocytes and human whole blood samples, but only at a high dosage of 1 mM. These differences may be due to differential nAChR expression, since it is demonstrated that nAChR expression on human leukocytes may vary due to genetic or environmental factors [6,7] or high nicotine exposure. Moreover, receptor affinities to nicotine are highly different amongst the different nAChR subclasses. Indeed, we observed that CHRNA7, CHRFAM7A and CHRNB2 expression varied between individuals.
Activating the so-called ‘cholinergic anti-inflammatory pathway’ via electrical vagus nerve stimulation, or the use of applied cholinergic agonists targeting distinct nAChR subtypes, could be a potential therapy in the treatment of inflammatory disorders in humans. However, a more physiological application, such as high-fat enteral nutrition, has been shown to activate this ‘cholinergic anti-inflammatory pathway’ as well [30]. High-fat enteral nutrition activates parasympathetic CCK-dependent vagal afferent pathways, which may converge to vagal efferent activation. In support of this mechanism, enteral nutrition with long-chain fatty acids in the form of olive oil suppresses LPS-induced TNF release of macrophages in the gut wall in mice (unpubl. data). Moreover, an extra-virgin olive oil-enriched diet (10%) showed protective effects in experimental mouse models of dextran sulfate sodium colitis and endotoxic shock [38,39]. In our study, olive oil administration reduced LPS-induced TNF production in whole blood samples of 4 healthy subjects, while it enhanced TNF production in 3 other volunteers. However, although it is presumed that olive oil stimulates the vagus nerve, we did not incorporate vagus nerve activity into our study. On the other hand, there is evidence that exposure to fat for only 3 min is sufficient to elicit a pancreatic polypeptide response [40]. Secretion of pancreatic polypeptide, a polypeptide secreted by pancreatic peptide cells in the endocrine pancreas, is regulated by cholinergic stimulation [41]. It remains to be established whether acetylcholine released from vagus nerve termini after olive oil administration actually reaches immune cells in the blood. However, olive oil administration did alter TNF production in the whole blood samples of 7 volunteers, indicating that olive oil can modulate the immune response in whole blood. Whether or not this is indeed mediated by the vagus nerve, and is responsible for the individual variation, needs to be further established.
In conclusion, we demonstrated that chronic exposure to nicotine enhances CHRNA7 expression on circulating monocytes in humans, in vitro and in vivo. Results obtained in a wide range of in vitro and in vivo models of inflammation imply that therapeutic agents targeting the cholinergic anti-inflammatory pathway can be an important asset in the treatment of immune disorders in humans. Our data indicate that there might be an individual variation in response to future therapeutic agents modifying the cholinergic anti-inflammatory pathway in humans.
Acknowledgments
The authors are thankful for grant support from TIPharma, grant 1-215 to E.P.v.d.Z., F.H., R.M.v.d.W. and W.J.d.J. G.E.B. was supported by an Odysseus grant of the Flemish ‘Fonds Wetenschappelijk Onderzoek’ (FWO). Further grant support is acknowledged from the Dutch Organization for Scientific Research (NWO; a VICI grant to G.E.B. and a VIDI grant to W.J.d.J.).
References
- 1.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 2.van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, LaRosa GJ, Ulloa L, Tracey KJ, van der Poll T. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 3.The FO, Boeckxstaens GE, Snoek SA, Cash JL, Bennink R, LaRosa GJ, van den Wijngaard RM, Greaves DR, de Jonge WJ. Activation of the cholinergic anti-inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterology. 2007;133:1219–1228. doi: 10.1053/j.gastro.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Van Maanen MA, Lebre MC, van der Poll T, LaRosa GJ, Elbaum D, Vervoordeldonk MJ, Tak PP. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 2009;60:114–122. doi: 10.1002/art.24177. [DOI] [PubMed] [Google Scholar]
- 5.Yu H, Yang YH, Rajaiah R, Moudgil KD. Nicotine-induced differential modulation of autoimmune arthritis in the Lewis rat involves changes in interleukin-17 and anti-cyclic citrullinated peptide antibodies. Arthritis Rheum. 2011;63:981–991. doi: 10.1002/art.30219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007;80:2314–2319. doi: 10.1016/j.lfs.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 7.Sato KZ, Fujii T, Watanabe Y, Yamada S, Ando T, Kazuko F, Kawashima K. Diversity of mRNA expression for muscarinic acetylcholine receptor subtypes and neuronal nicotinic acetylcholine receptor subunits in human mononuclear leukocytes and leukemic cell lines. Neurosci Lett. 1999;266:17–20. doi: 10.1016/s0304-3940(99)00259-1. [DOI] [PubMed] [Google Scholar]
- 8.van der Zanden EP, Snoek SA, Heinsbroek SE, Stanisor OI, Verseijden C, Boeckxstaens GE, Peppelenbosch MP, Greaves DR, Gordon S, de Jonge WJ. Vagus nerve activity augments intestinal macrophage phagocytosis via nicotinic acetylcholine receptor alpha4beta2. Gastroenterology. 2009;137:1029–1039. doi: 10.1053/j.gastro.2009.04.057. [DOI] [PubMed] [Google Scholar]
- 9.Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 11.Gault J, Robinson M, Berger R, Drebing C, Logel J, Hopkins J, Moore T, Jacobs S, Meriwether J, Choi MJ, Kim EJ, Walton K, Buiting K, Davis A, Breese C, Freedman R, Leonard S. Genomic organization and partial duplication of the human alpha7 neuronal nicotinic acetylcholine receptor gene (CHRNA7) Genomics. 1998;52:173–185. doi: 10.1006/geno.1998.5363. [DOI] [PubMed] [Google Scholar]
- 12.Benfante R, Antonini RA, De Pizzol M, Gotti C, Clementi F, Locati M, Fornasari D. Expression of the alpha7 nAChR subunit duplicate form (CHRFAM7A) is down-regulated in the monocytic cell line THP-1 on treatment with LPS. J Neuroimmunol. 2011;230:74–84. doi: 10.1016/j.jneuroim.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Villiger Y, Szanto I, Jaconi S, Blanchet C, Buisson B, Krause KH, Bertrand D, Romand JA. Expression of an alpha7 duplicate nicotinic acetylcholine receptor-related protein in human leukocytes. J Neuroimmunol. 2002;126:86–98. doi: 10.1016/s0165-5728(02)00057-7. [DOI] [PubMed] [Google Scholar]
- 14.de Lucas-Cerrillo AM, Maldifassi MC, Arnalich F, Renart J, Atienza G, Serantes R, Cruces J, Sanchez-Pacheco A, Andres-Mateos E, Montiel C. Function of partially duplicated human α77 nicotinic receptor subunit CHRFAM7A gene: potential implications for the cholinergic anti-inflammatory response. J Biol Chem. 2011;286:594–606. doi: 10.1074/jbc.M110.180067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandborn WJ, Tremaine WJ, Offord KP, Lawson GM, Petersen BT, Batts KP, Croghan IT, Dale LC, Schroeder DR, Hurt RD. Transdermal nicotine for mildly to moderately active ulcerative colitis: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1997;126:364–371. doi: 10.7326/0003-4819-126-5-199703010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Birrenbach T, Bocker U. Inflammatory bowel disease and smoking: a review of epidemiology, pathophysiology, and therapeutic implications. Inflamm Bowel Dis. 2004;10:848–859. doi: 10.1097/00054725-200411000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Ingram JR, Rhodes J, Collins PW, Williams GT, Newcombe RG, Thomas GA. Plasma fibrinogen in ulcerative colitis: the effect of disease activity and nicotine therapy in a randomised controlled trial. Dig Liver Dis. 2005;37:832–837. doi: 10.1016/j.dld.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Pullan RD, Rhodes J, Ganesh S, Mani V, Morris JS, Williams GT, Newcombe RG, Russell MA, Feyerabend C, Thomas GA, et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med. 1994;330:811–815. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- 19.Thomas GA, Rhodes J, Green JT, Richardson C. Role of smoking in inflammatory bowel disease: implications for therapy. Postgrad Med J. 2000;76:273–279. doi: 10.1136/pmj.76.895.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun M, Lee CJ, Shin HS. Reduced nicotinic receptor function in sympathetic ganglia is responsible for the hypothermia in the acetylcholinesterase knockout mouse. J Physiol. 2007;578:751–764. doi: 10.1113/jphysiol.2006.120147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122–1130. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Snoek SA, Verstege MI, van der Zanden EP, Deeks N, Bulmer DC, Skynner M, Lee K, Te Velde AA, Boeckxstaens GE, de Jonge WJ. Selective alpha7 nicotinic acetylcholine receptor agonists worsen disease in experimental colitis. Br J Pharmacol. 2010;160:322–333. doi: 10.1111/j.1476-5381.2010.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGrath J, McDonald JW, Macdonald JK. Transdermal nicotine for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2004:CD004722. doi: 10.1002/14651858.CD004722.pub2. [DOI] [PubMed] [Google Scholar]
- 24.De Rosa MJ, Esandi MC, Garelli A, Rayes D, Bouzat C. Relationship between alpha 7 nAChR and apoptosis in human lymphocytes. J Neuroimmunol. 2005;160:154–161. doi: 10.1016/j.jneuroim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Wongsriraksa A, Parsons ME, Whelan CJ. Characterisation of nicotine receptors on human peripheral blood mononuclear cells (PBMC) Inflamm Res. 2009;58:38–44. doi: 10.1007/s00011-008-8171-x. [DOI] [PubMed] [Google Scholar]
- 26.Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999;289:1545–1552. [PubMed] [Google Scholar]
- 27.Smeets AJ, Westerterp-Plantenga MS. Satiety and substrate mobilization after oral fat stimulation. Br J Nutr. 2006;95:795–801. doi: 10.1079/bjn20051725. [DOI] [PubMed] [Google Scholar]
- 28.Lubbers T, Luyer MD, De Haan JJ, Hadfoune M, Buurman WA, Greve JW. Lipid-rich enteral nutrition reduces postoperative ileus in rats via activation of cholecystokinin-receptors. Ann Surg. 2009;249:481–487. doi: 10.1097/SLA.0b013e318194d187. [DOI] [PubMed] [Google Scholar]
- 29.de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luyer MD, Greve JW, Hadfoune M, Jacobs JA, Dejong CH, Buurman WA. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med. 2005;202:1023–1029. doi: 10.1084/jem.20042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sykes AP, Brampton C, Klee S, Chander CL, Whelan C, Parsons ME. An investigation into the effect and mechanisms of action of nicotine in inflammatory bowel disease. Inflamm Res. 2000;49:311–319. doi: 10.1007/s000110050597. [DOI] [PubMed] [Google Scholar]
- 32.Severance EG, Dickerson FB, Stallings CR, Origoni AE, Sullens A, Monson ET, Yolken RH. Differentiating nicotine- versus schizophrenia-associated decreases of the alpha7 nicotinic acetylcholine receptor transcript, CHRFAM7A, in peripheral blood lymphocytes. J Neural Transm. 2009;116:213–220. doi: 10.1007/s00702-008-0164-y. [DOI] [PubMed] [Google Scholar]
- 33.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vida G, Pena G, Deitch EA, Ulloa L. Alpha7-cholinergic receptor mediates vagal induction of splenic norepinephrine. J Immunol. 2011;186:4340–4346. doi: 10.4049/jimmunol.1003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Damsgaard CT, Lauritzen L, Calder PC, Kjaer TM, Frokiaer H. Whole-blood culture is a valid low-cost method to measure monocytic cytokines – a comparison of cytokine production in cultures of human whole-blood, mononuclear cells and monocytes. J Immunol Methods. 2009;340:95–101. doi: 10.1016/j.jim.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Hamano R, Takahashi HK, Iwagaki H, Kanke T, Liu K, Yoshino T, Sendo T, Nishibori M, Tanaka N. Stimulation of adenosine A2A receptor inhibits LPS-induced expression of intercellular adhesion molecule 1 and production of TNF-alpha in human peripheral blood mononuclear cells. Shock. 2008;29:154–159. doi: 10.1097/shk.0b013e31812385da. [DOI] [PubMed] [Google Scholar]
- 37.Kox M, van Velzen JF, Pompe JC, Hoedemaekers CW, van der Hoeven JG, Pickkers P. GTS-21 inhibits pro-inflammatory cytokine release independent of the Toll-like receptor stimulated via a transcriptional mechanism involving JAK2 activation. Biochem Pharmacol. 2009;78:863–872. doi: 10.1016/j.bcp.2009.06.096. [DOI] [PubMed] [Google Scholar]
- 38.Leite MS, Pacheco P, Gomes RN, Guedes AT, Castro-Faria-Neto HC, Bozza PT, Koatz VL. Mechanisms of increased survival after lipopolysaccharide-induced endotoxic shock in mice consuming olive oil-enriched diet. Shock. 2005;23:173–178. doi: 10.1097/01.shk.0000148072.12094.77. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Fidalgo S, Villegas I, Cardeno A, Talero E, Sanchez-Hidalgo M, Motilva V, Alarcon de la Lastra C. Extra-virgin olive oil-enriched diet modulates DSS-colitis-associated colon carcinogenesis in mice. Clin Nutr. 2010;29:663–673. doi: 10.1016/j.clnu.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Simonian HP, Kresge KM, Boden GH, Parkman HP. Differential effects of sham feeding and meal ingestion on ghrelin and pancreatic polypeptide levels: evidence for vagal efferent stimulation mediating ghrelin release. Neurogastroenterol Motil. 2005;17:348–354. doi: 10.1111/j.1365-2982.2004.00634.x. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz TW. Pancreatic polypeptide: a hormone under vagal control. Gastroenterology. 1983;85:1411–1425. [PubMed] [Google Scholar]