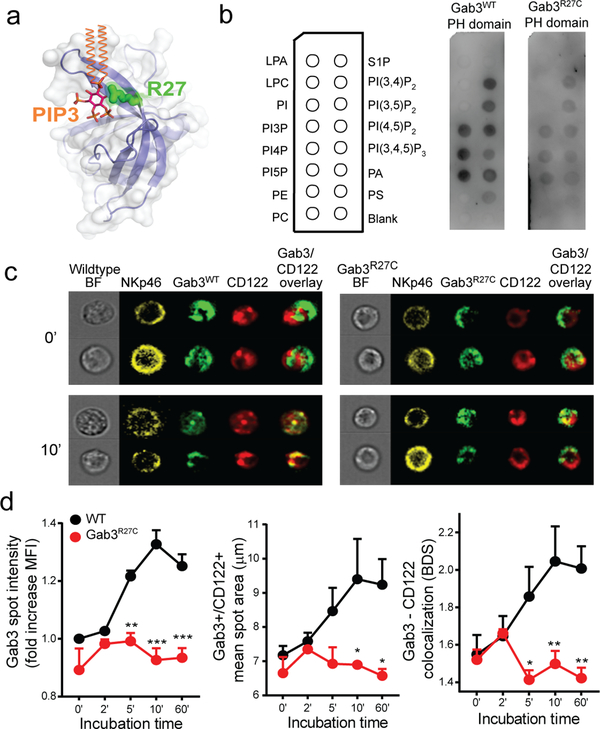
Figure 4. The R27C missense mutation disrupts Gab3 PH-domain binding to PIPs and impairs recruitment to the IL2-receptor complex.
a) 3D model of the PH-domain of WT Gab3 complexed with phosphatidylinositol-(3,4,5)-trisphosphate (PIP3) (orange & magenta). The Arg27 position (green) is predicted to interact with the phosphoinositide headgroup. b) PIP strips incubated with Gab3WT or Gab3R27C PH-domain. c) Representative images of Gab3KO NK cells transfected with Gab3WT-GFP or Gab3R27C-GFP fusion proteins to assess Gab3 colocalization with CD122 upon stimulation with IL-2. d) ImageStream colocalization studies between CD122 and Gab3WT-GFP or Gab3R27C-GFP following various incubation times with IL-2. Colocalization was defined by the intensity of Gab3 in CD122+ vesicles, the diameter and area of Gab3/CD122+ vesicles and colocalization assessed by bright detail similarity. ImageStream data represent mean values ± s.e.m of 3 experiments with >500 NK cells per experiment. A two-way ANOVA with Tukey post-test was performed to assess significance. *P<0.05, **P<0.01, ***P<0.001.