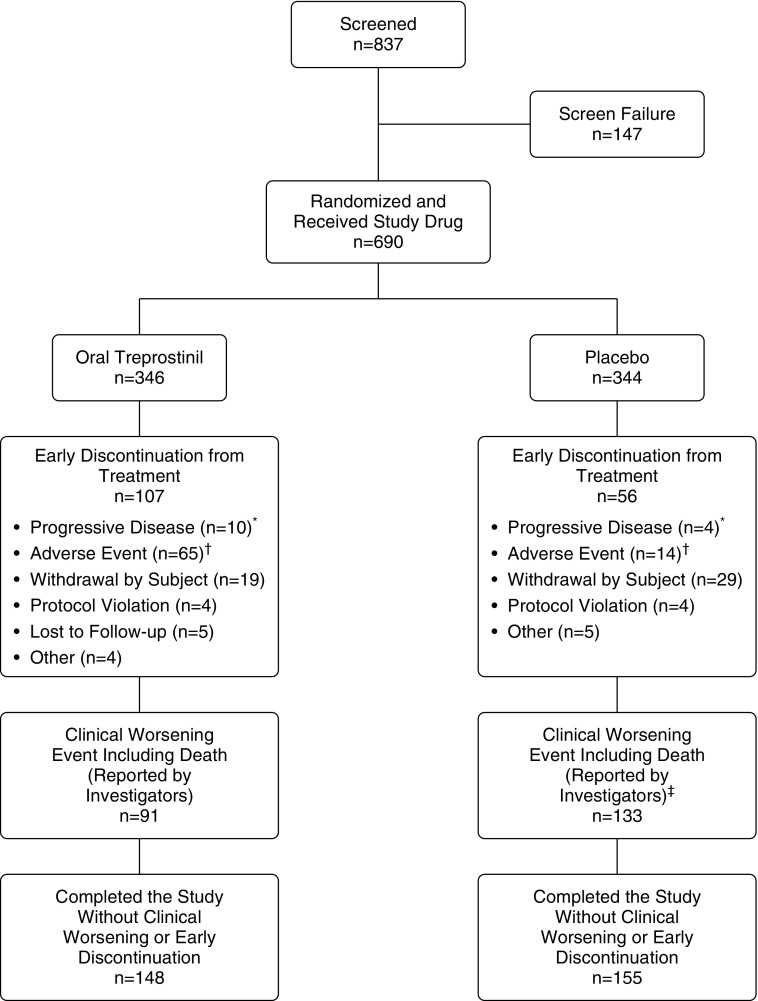
Figure 1.
Patient disposition. *Includes one subject in the oral treprostinil group and one subject in the placebo group who experienced clinical worsening events due to urgent hospitalization for treatment of worsening pulmonary arterial hypertension. †Includes one subject in the oral treprostinil group and one subject in the placebo group who experienced clinical worsening events due to fatal serious adverse events, and one subject in the oral treprostinil group who discontinued treatment due to an adverse event, but remained in the study until death (which did not qualify as a clinical worsening event). ‡Includes one subject in the placebo group who died after discontinuation of study treatment due to clinical worsening.