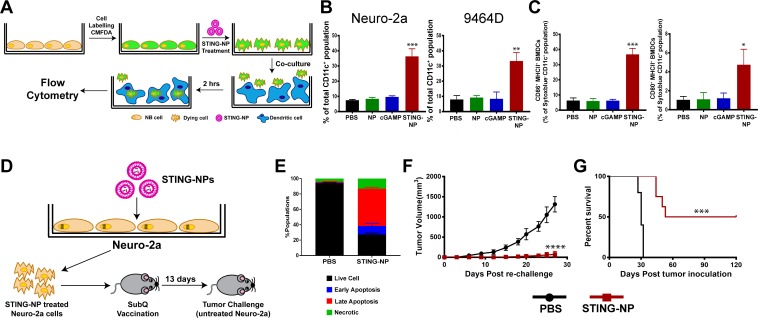
Figure 3.
Activation of STING in neuroblastoma increases tumor cell phagocytosis and generates protective antitumor immunity. (A) Schematic representation of tumor cell phagocytosis assay. (B) Flow cytometric quantification of relative NB cell uptake by BMDCs after co-culture with Neuro-2a or 9464D cells that were treated with PBS, empty NPs, 800 nM cGAMP, or STING-NPs and stained with CMFDA. (C) Percentage of CD11c+MHCII+CD86+ and CD11c+MHCII+ CD80+ BMDCs after 18 hours of co-culture with Neuro-2a cells treated with PBS, NPs, 800 nM cGAMP and STING-NPs; **p<0.01 and ***p<0.001, one-way ANOVA followed by Dunnett’s multiple comparison test. (D) Schematic representation of cell-based tumor vaccination model. A/J mice were vaccinated with Neuro-2a cells pre-treated with STING-NPs at 800 nM cGAMP for 48 hours (n=8 mice/group) or vehicle control (PBS, n=5 mice/group) and 13 days later mice were challenged with 106 live Neuro-2a cells in the opposite flank and tumor growth was measured. (E) Annexin V/7-AAD staining was used to confirm that~70% of NB cells were dying (~15% necrotic,~10% early apoptotic,~45% late apoptotic) prior to vaccination. Tumor growth curves (F) and percent survival (G) of naïve tumor-free mice that were vaccinated with Neuro-2a cells pre-treated with STING-NPs or vehicle control (PBS), and subsequently challenged with Neuro-2a cells on the opposite flank. Tumor volumes were compared on day 27 by Student’s t-test and log-rank (Mantel-Cox) test was used to compare Kaplan-Meier survival curves. ***p<0.001, ****p<0.0001 indicate a statistically significant difference. All data shown is mean±SD. ANOVA, analysis of variance; BMDCs, bone marrow-derived dendritic cells;cGAMP, cyclic guanosine monophosphate–adenosine monophosphate; NB, neuroblastoma; NP, nanoparticles;STING, stimulator of interferon genes; STING-NPs, STING-activating nanoparticles.