Table 3. Completing the flow synthesis of 1: Optimization of the BH3·DMS-mediated amide/ester reduction.
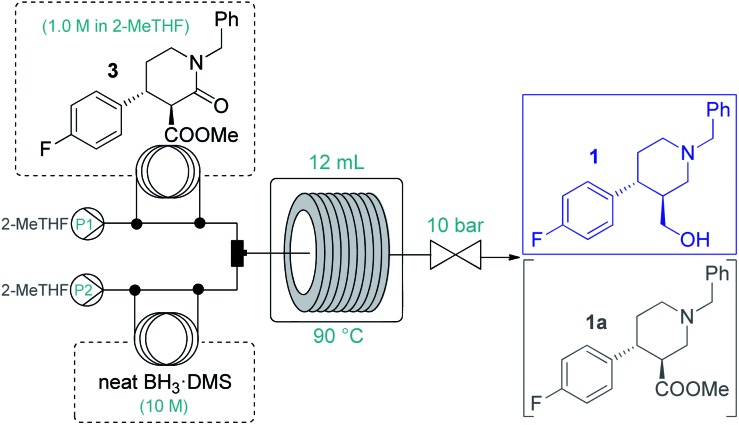
| |||||
| # a | Flow rate (μL min–1) |
3/BH3·DMS ratio | Conv. b (%) | Chemosel. b , c (%) | |
| P1 | P2 | ||||
| 1 d | 200 | 200 e | 1 : 10 | 96 | 74 |
| 2 | 100 | 100 f | 1 : 10 | 100 | 100 |
| 3 | 130 | 70 f | 1 : 5.4 | 100 | 100 |
| 4 | 140 | 60 f | 1 : 4.3 | 100 | 95 |
| 5 | 150 | 50 f | 1 : 3.3 | 99 | 76 |
| 6 | 260 | 140 e | 1 : 5.4 | 100 | 100 |
| 7 | 390 | 210 g | 1 : 5.4 | 100 | 93 |
a95-96% ee was measured in all reactions (by using chiral HPLC).
bDetermined by 1H-NMR analysis of the crude product.
c 1a formed as minor product in entries 1, 4, 5 and 7.
dAt 50 °C.
e t r= 30 min.
f t r= 60 min.
g t r= 20 min.
