Abstract
Background
Acne scarring is a frequent complication of acne and resulting scars may negatively impact on an affected person's psychosocial and physical well‐being. Although a wide range of interventions have been proposed, there is a lack of high‐quality evidence on treatments for acne scars to better inform patients and their healthcare providers about the most effective and safe methods of managing this condition. This review aimed to examine treatments for atrophic and hypertrophic acne scars, but we have concentrated on facial atrophic scarring.
Objectives
To assess the effects of interventions for treating acne scars.
Search methods
We searched the following databases up to November 2015: the Cochrane Skin Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (2015, Issue 10), MEDLINE (from 1946), EMBASE (from 1974), and LILACS (from 1982). We also searched five trials registers, and checked the reference lists of included studies and relevant reviews for further references to randomised controlled trials.
Selection criteria
We include randomised controlled trials (RCTs) which allocated participants (whether split‐face or parallel arms) to any active intervention (or a combination) for treating acne scars. We excluded studies dealing only or mostly with keloid scars.
Data collection and analysis
Three review authors independently extracted data from each of the studies included in this review and evaluated the risks of bias. We resolved disagreements by discussion and arbitration supported by a method expert as required. Our primary outcomes were participant‐reported scar improvement and any adverse effects serious enough to cause participants to withdraw from the study.
Main results
We included 24 trials with 789 adult participants aged 18 years or older. Twenty trials enrolled men and women, three trials enrolled only women and one trial enrolled only men. We judged eight studies to be at low risk of bias for both sequence generation and allocation concealment. With regard to blinding we judged 17 studies to be at high risk of performance bias, because the participants and dermatologists were not blinded to the treatments administered or received; however, we judged all 24 trials to be at a low risk of detection bias for outcome assessment. We evaluated 14 comparisons of seven interventions and four combinations of interventions. Nine studies provided no usable data on our outcomes and did not contribute further to this review's results.
For our outcome 'Participant‐reported scar improvement' in one study fractional laser was more effective in producing scar improvement than non‐fractional non‐ablative laser at week 24 (risk ratio (RR) 4.00, 95% confidence interval (CI) 1.25 to 12.84; n = 64; very low‐quality evidence); fractional laser showed comparable scar improvement to fractional radiofrequency in one study at week eight (RR 0.78, 95% CI 0.36 to 1.68; n = 40; very low‐quality evidence) and was comparable to combined chemical peeling with skin needling in a different study at week 48 (RR 1.00, 95% CI 0.60 to 1.67; n = 26; very low‐quality evidence). In a further study chemical peeling showed comparable scar improvement to combined chemical peeling with skin needling at week 32 (RR 1.24, 95% CI 0.87 to 1.75; n = 20; very low‐quality evidence). Chemical peeling in one study showed comparable scar improvement to skin needling at week four (RR 1.13, 95% CI 0.69 to 1.83; n = 27; very low‐quality evidence). In another study, injectable fillers provided better scar improvement compared to placebo at week 24 (RR 1.84, 95% CI 1.31 to 2.59; n = 147 moderate‐quality evidence).
For our outcome ‘Serious adverse effects’ in one study chemical peeling was not tolerable in 7/43 (16%) participants (RR 5.45, 95% CI 0.33 to 90.14; n = 58; very low‐quality evidence).
For our secondary outcome ‘Participant‐reported short‐term adverse events’, all participants reported pain in the following studies: in one study comparing fractional laser to non‐fractional non‐ablative laser (RR 1.00, 95% CI 0.94 to 1.06; n = 64; very low‐quality evidence); in another study comparing fractional laser to combined peeling plus needling (RR 1.00, 95% CI 0.86 to 1.16; n = 25; very low‐quality evidence); in a study comparing chemical peeling plus needling to chemical peeling (RR 1.00, 95% CI 0.83 to 1.20; n = 20; very low‐quality evidence); in a study comparing chemical peeling to skin needling (RR 1.00, 95% CI 0.87 to 1.15; n = 27; very low‐quality evidence); and also in a study comparing injectable filler and placebo (RR 1.03, 95% CI 0.10 to 11.10; n = 147; low‐quality evidence).
For our outcome ‘Investigator‐assessed short‐term adverse events’, fractional laser (6/32) was associated with a reduced risk of hyperpigmentation than non‐fractional non‐ablative laser (10/32) in one study (RR 0.60, 95% CI 0.25 to 1.45; n = 64; very low‐quality evidence); chemical peeling was associated with increased risk of hyperpigmentation (6/12) compared to skin needling (0/15) in one study (RR 16.00, 95% CI 0.99 to 258.36; n = 27; low‐quality evidence). There was no difference in the reported adverse events with injectable filler (17/97) compared to placebo (13/50) (RR 0.67, 95% CI 0.36 to 1.27; n = 147; low‐quality evidence).
Authors' conclusions
There is a lack of high‐quality evidence about the effects of different interventions for treating acne scars because of poor methodology, underpowered studies, lack of standardised improvement assessments, and different baseline variables.
There is moderate‐quality evidence that injectable filler might be effective for treating atrophic acne scars; however, no studies have assessed long‐term effects, the longest follow‐up being 48 weeks in one study only. Other studies included active comparators, but in the absence of studies that establish efficacy compared to placebo or sham interventions, it is possible that finding no evidence of difference between two active treatments could mean that neither approach works. The results of this review do not provide support for the first‐line use of any intervention in the treatment of acne scars.
Although our aim was to identify important gaps for further primary research, it might be that placebo and or sham trials are needed to establish whether any of the active treatments produce meaningful patient benefits over the long term.
Plain language summary
Treatment for acne scars
Review question
Which treatments are effective for acne scars?
Background
Acne scars may have a damaging effect on a person's physical, mental, and social well‐being. Although a wide range of treatments are used, there is a lack of high‐quality evidence on which are the most effective for acne scars.
This review aimed to better inform patients and healthcare providers about the most effective and safe methods to manage this problem. We have examined treatments for atrophic scars (depressions in the skin surface) and hypertrophic scars (lumpy scars that stick out from the skin surface) in acne but have concentrated on facial atrophic scarring. Our main outcomes of interest were participant‐reported scar improvement and any adverse effects serious enough to cause participants to withdraw from the study.
Study characteristics
We include 24 randomised controlled trials (RCTs) with 789 people with acne scars (from searches up to November 2015). Twenty‐one RCTs (706 people) enrolled both men and women, three RCTs (75 people) enrolled only women and one RCT (eight people) enrolled only men. Most of the studies we included (21 RCTs with 744 people) enrolled people with atrophic acne scars. One RCT enrolled 20 individuals with mixed atrophic and hypertrophic acne scars.
Key results
There is insufficient evidence from trials to support fractional laser for treatment of acne. However, this management approach is adopted by some in clinical practice for the treatment of acne scarring.
For our outcome 'Participant‐reported scar improvement' fractional laser was more effective in producing scar improvement change than non‐fractional non‐ablative laser. Fractional radiofrequency showed similar scar improvement to fractional laser. Chemical peeling showed similar scar improvement to both fractional laser and skin needling. Combined chemical peeling with skin needling showed similar scar improvement to fractional laser and to deep chemical peeling. Injectable fillers provided better scar improvement compared to placebo.
Our outcome ‘Serious adverse effects’ was reported in one study, showing that chemical peeling was not tolerable in 16% of those taking part. Other outcomes, ‘Participant‐reported' and 'Investigator‐assessed' adverse events in the short term (less than 24 weeks), were more or less acceptable by those taking part and by investigators and did not reveal a big difference between the studied interventions.
Four out of six of our comparisons were completely inconclusive and they were of very low‐quality evidence. There is a lack of studies that establish efficacy of treatments compared to placebo or sham interventions, and it is possible that finding no evidence of difference between two active treatments could mean that neither is very useful.
We did not identify any trials that examined treatment for acne scars on the back.
The results of this review do not support the first‐line use of any intervention in the treatment of acne scars, and no studies provided evidence to confirm that any short‐term benefit will translate to long‐term effects.
Quality of the evidence
We rated the quality of the evidence for several outcomes as very low to moderate. The lower quality evidence for treatments was mostly because there were few people in the studies, making the results less precise, and there was a lack of blinding (people knew the treatment they were receiving).
Future studies should consider adopting patient‐reported outcomes as a primary measure. There should be a set of core outcome measures reported in all RCTs for treating acne scars, and outcomes should be evaluated several months after the treatment has been done. Lack of reporting of serious side effects was one of the research gaps found in this review.
Summary of findings
Background
A description of key medical terms can be found in Appendix 1.
Description of the condition
Acne and its prevalence
Acne vulgaris is a chronic inflammatory condition of the pilosebaceous unit (Fabbrocini 2010; Williams 2012). It is notable for open or closed comedones or both and for inflammatory lesions including papules, pustules, or nodules (Strauss 2007). Acne vulgaris is among the top 10 most prevalent conditions worldwide (Hay 2014), and it is one of the most common skin conditions. Some degree of acne affects almost all adolescents between 15 and 17 years of age (Collier 2007; Williams 2012). Up to 80% of adolescents and up to 5% of adults experience acne (Jacob 2001).
The impact of acne on the quality of life can be profound (Dalgard 2009). Compared to people without acne, individuals with acne are at a higher risk of experiencing depression and anxiety, especially in those whose quality of life has been affected (Duman 2015). They are more likely to have lower self esteem and lower body satisfaction and may be at an increased risk of suicide attempts (Purvis 2006). Optimal treatments may significantly improve the appearance, quality of life, and self esteem of affected people (Purvis 2006).
It is important for dermatologists to be aware of a condition known as dysmorphophobia or body dysmorphic disorder, which can affect people with acne and acne scars. It is characterised by a distressing and excessive preoccupation with a slight or imagined defect of a physical feature and may significantly damage psychosocial functioning and decrease the quality of life of those affected (Conrado 2010; Gupta 2013).
Acne scars: aetiology, pathology, and prevalence
Scarring, as a physical disfigurement, is a frequent complication of acne. The psychological impact of scarring can be profound; scars can occur as a result of damage to the skin during the healing of active acne (Patel 2010). Although active acne can persist for a decade or more, acne scars may persist for a lifetime (Jordan 2000). One publication (Layton 1994) on the prevalence of acne scarring suggests that the type and extent of scarring correlates with the site and severity of previous acne and duration of acne before effective treatment. Facial scarring affects both sexes equally and occurs in up to 95% of cases (Layton 1994).
Several classifications and scales have been proposed for facial acne scarring (Dreno 2007; Goodman 2006a; Goodman 2006b).
Often, scarring is the consequence of severe inflammatory nodulocystic acne, but it may also be the product of superficial inflamed lesions or the squeezing or picking of lesions with the fingernails (Patel 2010). There are three general types of acne scars, depending on hyperproliferation or loss of collagen: hypertrophic scars, keloid scars or atrophic scars. A person might have one or more types occurring in the same skin area (Basta‐Juzbasic 2010; Maibach 2011).
Atrophic scars are seen in almost 80% to 90% of patients (Patel 2010). These scars present clinically as indentations in the skin due to loss of collagen and destructive inflammation in the deep dermis with subsequent contraction (Jacob 2001). Atrophic scars may be further classified into ice pick scars (V‐shaped epithelial tracts with a sharp margin that can extend deeper in the skin), boxcar scars (a round to oval scar with sharp vertical sides that can extend deeper in the skin), or rolling scars (irregular scars with a rolling or undulating shape that may reach up to 5 mm in diameter) occurring in 60% to 70%, 20% to 30%, and 15% to 25% of patients respectively (Jacob 2001). These three scar types are usually seen in the same person, making it difficult to differentiate between them (Lee 2009; Levy 2012).
Limited morphological classification of scarring has been described and to date there is poor consensus; clinical assessment of scars demonstrates significant variation between assessors (Finlay 2013). The lack of a universally accepted standardised objective quantification or qualitative scoring to estimate the global severity and burden of disease makes comparisons of treatments for scarring challenging. There is some evidence for differences in innate immune responses in those that scar and do not scar (Holland 2004); this makes it difficult to interpret results between participants.
Hypertrophic and keloidal scars show excessive deposition of collagen with reduced collagenase activity (Alster 2003). Individuals with Type IV/V Fitzpatrick skin types are more liable to develop hypertrophic or keloid scars, and both scars predominantly occur on the trunk (Brown 2009). Typically, hypertrophic scars are raised firm pink lesions that remain within the borders of the original site of injury (Gauglitz 2011). In contrast, keloids appear reddish‐purple and take the form of papules and nodules usually extending beyond the borders of the original wound (Gauglitz 2011). Keloids do not tend to regress spontaneously and are frequently resistant to treatment and have a high recurrence rate (Brown 2009; Fabbrocini 2010; Lee 2009).
The destructive treatments that we consider in this review can worsen keloids (Mutalik 2005). We therefore exclude management of keloidal scars from this review, so this will need to be addressed in a separate review.
Description of the intervention
The management of acne scarring includes various types of resurfacing (chemical peels, lasers, dermabrasion); use of injectable fillers; and also surgical methods, such as needling, subcision, punch excision, or punch elevation (Basta‐Juzbasic 2010; Cao 2015; Fabbrocini 2010). Different factors, e.g. colour, texture, and morphology, can affect the treatment choice for each individual scar (Basta‐Juzbasic 2010). Combining interventions may produce more benefit compared with a single method alone. Complete resolution of acne scars can not be achieved by the currently available treatment modalities (Basta‐Juzbasic 2010). Early effective treatment of acne is probably the best strategy to prevent or limit post‐acne scarring (Goodman 2014; Williams 2012).
Different interventions for the treatment of acne scars sometimes entail a significant cost (Jordan 2000). The costs for the same intervention sometimes vary considerably between different countries or regions.
How the intervention might work
Traditional ablative laser resurfacing removes the epidermis and part of the dermis of the scars, allowing collagen remodeling and re‐epithelialisation (Jordan 2000). Patients typically do not need more than one treatment, but the treatment has adverse effects including persistent erythema, hypopigmentation, hyperpigmentation, infections, and scarring. It also has a recovery period (up to two weeks) (Goodman 2014). Proper training is required for performing ablative laser resurfacing (Goodman 2014).
Non‐ablative laser resurfacing produces dermal thermal injury while preserving the epidermis; this dermal thermal injury promotes collagen remodeling through the formation of new collagen, which leads to an improvement in the scarring (Hedelund 2010).
Fractional laser resurfacing acts, as the name indicates, "on regularly‐spaced arrays over a fraction of the skin surface to induce thermal ablation of microscopic columns of epidermal and dermal tissue" (Goel 2011). This approach is more effective than non‐ablative resurfacing while providing a faster recovery when compared with ablative resurfacing (Alexiades‐Armenakas 2008). Fractional and non‐ablative laser resurfacing have become more popular in practice than ablative laser resurfacing, despite a non‐comparable efficacy, probably because of a lower rate of adverse events (Alexiades‐Armenakas 2008).
Chemical peels (employing glycolic acid, phenol, salicylic‐mandelic acid, or trichloroacetic acid) are used in treating small depressed scars but not ice pick or deep boxcar scars (Garg 2008; Garg 2014). A combination of chemical peeling, subcision, and microneedling may result in a better outcome (Fabbrocini 2010). However, excessive systemic absorption of phenolic chemical peels might increase the risk of cardiac toxicity (Landau 2007).
Dermabrasion involves the use of tools (e.g. high‐speed brush, diamond cylinder, fraise, or silicon carbide sandpaper) to remove the epidermis or epidermis and part of the dermis. An advantage of the procedure is that it allows the clinician to etch scar edges precisely without thermal injury. It may be effective for some acne scars, but is usually not used for ice pick or deep boxcar scars (Goodman 2014). Adverse effects include significant pain and a considerable recovery time. Scarring, pigment alterations, and milia formation can also occur with dermabrasion (Goodman 2014).
Skin‐needling procedures may diminish the appearance of acne scars. A needling device is rolled over the surface of the skin to form numerous perforations in the epidermis and dermis, with a goal of stimulating new collagen (Alam 2014). Needling therapy has been associated with improvement of dermatologist‐rated acne scarring (Alam 2014). The advantages of skin needling include low cost, a relatively short recovery period (two to three days), and a very low risk for postinflammatory hyperpigmentation (Fabbrocini 2009). Skin needling treatment is well tolerated by most people and the pain is minimal (Alam 2014).
Punch excision may be an effective treatment for ice pick scars and small (< 3 mm) boxcar scars. A punch biopsy instrument of equal to or slightly greater diameter than the scar is used to incise the tissue to the subcutaneous fat layer and excise the scar (Grevelink 1998). It has been associated with good results, but secondary widening of the scar may occur (Goodman 2014).
Punch elevation is best suited for boxcar scars (Goodman 2007). The scar border is excised, leaving the deepest part of the scar that is adherent to the fat layer. The scar is raised higher than the surrounding skin; it then retracts during healing to become level with the surface (Goodman 2014).
Subcision is used for the management of rolling or depressed scars; a blade inserted parallel to the skin surface is used to cut fibrotic strands tethering the scar to the underlying tissue (Jacob 2001). Reported adverse effects include bruising and swelling, bleeding, and infection (Alam 2005).
Injectable filler injections used for atrophic scars have been proposed to improve the appearance of acne scars; collagen, autologous fat transfer, and artificial injectable fillers are most commonly used (Karnik 2014). Their effect lasts from three to 18 months, depending on the type of filler used (Karnik 2014).
Hypertrophic scars are classically treated with intralesional corticosteroid injections (Arno 2014). Using multiple treatment methods gives the maximum potential for success, including earlier use of 5‐fluorouracil (Mutalik 2005). Clinical research increasingly supports the use of newer agents (e.g. bleomycin, onion extract, imiquimod, mitomycin C) and laser therapy (pulsed‐dye, fractional) for this type of scar management (Gold 2014).
Why it is important to do this review
Acne scars may cause important detrimental effects on a person's physical, mental, and social well‐being (Purvis 2006). Although a wide range of interventions have been proposed in this field, there is a lack of high‐quality synthesised evidence on interventions for acne scars to better inform caregivers and consumers about the most effective and safe methods to manage this problem.
Treatment of acne scars is among the top 10 research priorities for the treatment of acne identified by the Acne Priority Setting Partnership. The Acne Priority Setting Partnership was set up to identify and rank treatment uncertainties by bringing together consumers and professionals who provide care within and beyond the National Health Service (NHS) (Layton 2015). The National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme and its initiative to support the current review will use knowledge gaps identified by the review to inform areas of future research.
We have built on and expanded the work previously published in a Cochrane review that assessed laser resurfacing for facial acne scars (Jordan 2000). We have taken into account the uncertainties identified by that review.
Given the physical disfigurement associated with acne vulgaris, along with the potentially profound psychological impact of this skin disorder, we think it is necessary to assess the evidence on the benefits and harms of available treatments for acne scars. We are interested in acne scars on both face and back, but we have concentrated in this review on facial scars.
The plans for this review were published in the protocol 'Interventions for acne scars' (Abdel Hay 2015)
Objectives
To assess the effects of interventions for treating acne scars.
Methods
Criteria for considering studies for this review
Types of studies
We include randomised controlled trials (RCTs) allocating participants (whether by split‐face or parallel study designs) to any active intervention (or a combination) for treating acne scars. In multi‐arm trials, we have included all eligible arms.
We exclude cluster trials, cross‐over trials, and quasi‐RCTs.
Types of participants
People of either gender, all ages, and all ethnic groups who had been diagnosed by a dermatologist or an experienced investigator as having atrophic or hypertrophic acne scars. We include all grades of scar severity.
We exclude studies dealing with only or mostly keloid scars, because the destructive treatments that we consider in this review may worsen keloids.
Types of interventions
We include all interventions versus an active intervention, placebo, or no treatment. We consider all active interventions, including chemical peeling, dermabrasion and microdermabrasion, laser therapy, radiofrequency, punch techniques and dermal grafting, tissue‐augmenting agents, needling, subcision, intralesional steroid injection, silicone gel, cryotherapy, pulsed dye laser, imiquimod, 5‐fluorouracil, interferon, bleomycin, surgery, or combined therapy.
Types of outcome measures
Primary outcomes
Participant‐reported scar improvement: measured by a scar improvement, grading, or severity scale.
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study. We define 'serious' adverse effects' as events that pose a threat to a participant's life or functioning whereas 'severe' adverse effects are defined by their intensity.
Secondary outcomes
Investigator‐assessed scar improvement: measured by a scar improvement, grading, or severity scale.
Participant satisfaction: measured by a participant satisfaction questionnaire.
Quality of life: measured by a quality‐of‐life scale, whether global or specific.
Participant‐reported adverse events, e.g. pain, erythema, oedema, infection, oozing, crusting, hyperpigmentation, or scarring.
Investigator‐assessed adverse events, e.g. erythema, oedema, infection, oozing, crusting, hyperpigmentation, or scarring.
Duration, in days, of post‐procedure down time. We defined the down time as the number of days following the procedure during which the participant had oedema and erythema and felt unable or unwilling to go out in public.
Timing of outcomes
We assessed our primary outcome of scar improvement over a time frame of up to 24 weeks (short‐term) and more than 24 weeks (long‐term).
We assessed our adverse events outcomes in the short term up to four weeks after the procedure and in the long term more than four weeks after the procedure.
Search methods for identification of studies
We aimed to identify all relevant RCTs, regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following databases up to 18 November 2015:
the Cochrane Skin Group Specialised Register using the following terms: acne and (cicatri* or scar*);
the Cochrane Central Register of Controlled Trials (CENTRAL) 2015, Issue 10, in the Cochrane Library using the search strategy in Appendix 2;
MEDLINE via Ovid (from 1946) using the strategy in Appendix 3;
EMBASE via Ovid (from 1974) using the strategy in Appendix 4;
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the strategy in Appendix 5.
Trials registers
We searched the following trials registers on 24 December 2015, using the following terms: "acne" and (cicatri* or scar*).
The ISRCTN registry (www.isrctn.com/).
The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov).
The Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
The World Health Organization International Clinical Trials Registry platform (apps.who.int/trialsearch).
The EU Clinical Trials Register (www.clinicaltrialsregister.eu).
Searching other resources
We checked the bibliographies of included trials and review articles for further references to relevant trials.
We searched manufacturers' websites for relevant trial information.
We contacted trial authors for missing data and information about ongoing trials.
Adverse effects
We did not perform a separate search for adverse effects of interventions used for treating acne scars. We considered adverse effects described in the included studies only.
Data collection and analysis
Some of the data collection and analysis section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Review Group.
Selection of studies
We downloaded all potentially relevant studies identified from the searches into reference management software and removed duplicates. Two review authors independently screened titles and abstracts of studies from literature searches for inclusion in the review and coded them as "retrieve" (eligible, potentially eligible, or unclear) or "do not retrieve". We obtained the full texts of those coded "retrieve", and two review authors independently screened the full texts to identify studies for inclusion. We did not include studies reported as abstracts only, as we could not extract enough information for 'Risk of bias' assessment and completion of the Characteristics of included studies tables. We resolved disagreements by discussion, and if we did not reach consensus a third author made the judgement.
We recorded reasons for the exclusion of any ineligible studies in the 'Characteristics of excluded studies' tables.
We carried out the selection process in sufficient detail to complete a study flow diagram.
Data extraction and management
We used the data extraction form available from the Cochrane Skin Group's website and developed a computer database tool to be used for data extraction. We piloted the data extraction form within the review team using a sample of the studies to be reviewed. For eligible studies, two review authors independently extracted the data using the agreed form and then entered the data into Review Manager 5 software (RevMan 2014). We cross‐checked the data for accuracy. We resolved discrepancies through discussion or if required we consulted a third author. When information regarding any of the above was unclear, we contacted the authors of the original reports to elicit further details.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or with the involvement of a third author.
(1) Sequence generation (checking for possible selection bias)
We describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the risk as one of the following:
low (any truly random process, e.g. random number table, computer random number generator);
high (non‐random approach, e.g. sequence generated by odd or even date of birth, sequence generated by some rule based on date of admission); or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We describe for each included study the method used to conceal the allocation sequence in sufficient detail to determine whether allocation of the intervention could have been foreseen in advance of or during recruitment, or changed after assignment.
We assessed the risk as one of the following:
low (e.g. telephone or central randomisation, consecutively‐numbered sealed opaque envelopes);
high (open random allocation, unsealed or non‐opaque envelopes); or
unclear.
(3) Blinding (checking for possible performance and detection bias)
We describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant would receive. We assessed blinding separately for performance and detection bias and for different outcomes or classes of outcomes.
We assessed the risk as one of the following:
low, high, or unclear for participants;
low, high, or unclear for personnel; and
low, high, or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We describe for each included study and for each outcome or class of outcomes the completeness of data, including attrition and exclusions from the analysis. We considered both the overall attrition rate (the proportion of participants randomly assigned to the study groups for whom outcome data are not available) and the differential attrition rate (the difference in attrition rates between groups). We considered an overall attrition rate above 20% or a differential attrition rate above 5% as representing a high risk of attrition bias.
We state whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups. Where sufficient information was reported or could be supplied by the trial authors, we re‐included missing data in the analyses that we undertook. We assessed risk as one of the following:
low;
high; or
unclear.
(5) Selective reporting bias
We describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found, on the basis of what was present in the trial registry documents. We wrote to authors to ask for protocols if these were not published.
We assessed the risk as follows:
low, where it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review were reported;
high, where not all of the study's prespecified outcomes were reported, one or more reported primary outcomes were not prespecified, outcomes of interest were reported incompletely and so could not be used, or the study failed to include results of a key outcome that could be expected to be reported; or
unclear.
(6) Other sources of bias
We describe for each included study any important concerns that we have about other possible sources of bias, e.g. baseline imbalance and blocked randomisation in unblinded trials.
We assessed the risk as one of the following:
low;
high; or
unclear.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (one) to (six) above, we assessed the likely magnitude and direction of the bias and whether we considered that it was likely to impact on the treatment effects. We explore the impact of the level of bias through undertaking sensitivity analyses.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as summary risk ratios (RRs) with 95% confidence intervals (CIs).
Continuous data
For continuous data, we use the mean difference (MD) if outcomes were measured in the same way across trials. We use the standardised mean difference (SMD) to combine trials that measured the same outcome using different scales. We present change data and endpoint data separately in cases where we used the SMD.
Unit of analysis issues
We anticipated that the trials included in this review might randomise either participants or split‐face. We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We intended to incorporate trials using a split‐face design to approximate a paired analysis using the generic inverse variance method, and to undertake a sensitivity analysis where we had used imputations and to perform meta‐analyses of these trials separately from parallel trials. However paired data were unavailable and we were not able to adjust for the within‐individual variability. We report those studies separately as a RR without a P value or 95% CI. We include two multi‐arm trials in the review, using the two arms that compared different interventions for acne scars. We include these studies as pair‐wise comparisons.
Dealing with missing data
For all outcomes, we attempted to conduct analyses on an intention‐to‐treat basis. When there were missing data, we contacted the authors of the study to obtain the relevant missing data (See Table 8). We carefully evaluated important numerical data. If we could not obtain missing data for dichotomous outcomes, we considered participants with missing outcome data as treatment failures. We used sensitivity analysis to assess how sensitive results were to reasonable changes in the assumptions that we made. We addressed the potential impact of missing data on the findings of the review in the Discussion section.
1. Contacted authors.
| Study | Contact author | Contact email | Reply/did not reply |
| Ahmed 2014 | Mohammed G | dr_ghada77@hotmail.com | Did not reply |
| Asilian 2011 | Salimi E | s_salimi@resedent.mui.ac.ir | Did not reply |
| Bernstein 2001 | Bernstein EF | dermguy@hotmail.com | Did not reply |
| Chae 2015 | Choi YS | uuhderma@hanmail.net | Responded |
| Cho 2010 | Kim DH | terios92@hanmail.net | Did not reply |
| Gadkari 2014 | Gadkari R | drreshmagadkari@gmail.com | Did not reply |
| Hedelund 2010 | Hedelund L | lene.hedelund@hotmail.com | Did not reply |
| Hedelund 2012 | Hedelund L | lenhed@rm.dk | Did not reply |
| Karnik 2014 | Smith SR | ssmith@stacyrsmithmd.com | Did not reply |
| Kim 2009 | Lee JH | juhee@yuhs.ac | Did not reply |
| Kim 2009a | Kim S | i4ks@yahoo.com | Did not reply |
| Lee 2009 | Suh DH | daehun@snu.ac.kr | Did not reply |
| Lee 2011 | Kim BJ | beomjoon@unitel.co.kr | Did not reply |
| Linkner 2014 | Linkner RV | Rita.Linkner@mountsinai.org | Did not reply |
| Manuskiatti 2013 | Manuskiatti W | woraphong.man@mahidol.ac.th | Did not reply |
| Min 2009 | Suh DH | daehun@snu.ac.kr | Did not reply |
| Mohammed 2013 | Mohammed G | dr_ghada77@hotmail.com | Did not reply |
| Munavalli 2013 | Munavalli GS | gmunavalli@carolinaskin.com | Did not reply |
| Nofal 2014 | Nofal E | ahmadnofal5@hotmail.com | Did not reply |
| Rongsaard 2014 | Rongsaard N | nopnarueporn@gmail.com | Responded |
| Sage 2011 | Kouba DJ | dkouba1@hfhs.org | Did not reply |
| Tanzi 2004 | Alster TS | talster@skinlaser.com | Did not reply |
| Zhang 2013 | Chen J | xdchen@medmail.com.cn | Did not reply |
Assessment of heterogeneity
We assessed heterogeneity by considering clinical factors (type of scars, severity of scars, and skin phototype) and methodological factors (allocation concealment and attrition).
We tested statistical heterogeneity using the Chi² test (significance level: 0.1) and I² statistic (0% to 40%: may not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; 75% to 100%: represents considerable heterogeneity) (Higgins 2003; Higgins 2011).
Where we observed high levels of heterogeneity among the trials (I² statistic ≥ 50% or P < 0.1), we considered clinical factors (e.g. type of scars) and methodological factors (e.g. allocation concealment and attrition) of the included studies. We tried to explore the source of heterogeneity by subgroup analysis (described in Subgroup analysis and investigation of heterogeneity) or by Sensitivity analysis.
Assessment of reporting biases
We planned to investigate reporting biases (such as publication bias) for primary outcomes using funnel plots if there were 10 or more studies in the meta‐analysis. We planned to assess funnel plot asymmetry visually. If a visual assessment suggested asymmetry, we planned to perform exploratory sensitivity analyses.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (RevMan 2014). We used a fixed‐effect meta‐analysis for combining data from published studies where it was reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials were examining the same intervention and we judged the trials' populations and methods as sufficiently similar.
If there were clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if we had detected a high level of heterogeneity among the trials (I² statistic ≥ 50% or P < 0.1), we would have used a random‐effects meta‐analysis to produce an overall summary if we considered an average treatment effect across trials as clinically meaningful. We treated the random‐effects summary as the average of the range of possible treatment effects, and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. If we had used random‐effects analyses, we would have presented the results as the average treatment effects with 95% confidence intervals and the estimates of Tau² and I² statistic. If heterogeneity was considerable (I² statistic of 75% to 100%), we would not perform a meta‐analysis. Instead, we would have provided a narrative, qualitative summary.
For individual studies with low numbers of outcomes (fewer than 10 in total) or where the total sample size was less than 30 participants and a risk ratio was used, we reported the proportion of outcomes in each treatment group together with a P value from a Fisher's exact test (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses for our primary outcomes:
Type of scar: ice pick versus boxcar versus rolling versus hypertrophic;
Scar severity: superficial to medium scars versus deep scars;
Skin phototype: skin phototype I to III versus skin phototype IV to VI.
There were not enough (at least 10) studies to conduct the planned subgroup analysis. In future updates, we plan to conduct the prespecified subgroup analyses classifying whole trials by interaction tests (Higgins 2011).
Sensitivity analysis
We did not perform any sensitivity analysis, due to the paucity of studies for each comparison. In future updates, we plan to perform sensitivity analyses for assessing the quality of studies (by including studies judged to be at low risk of bias in allocation concealment and attrition domains). We also plan to carry out sensitivity analysis to explore the effects of fixed‐effect or random‐effects analyses for outcomes with statistical heterogeneity, and if we identify an asymmetrical funnel plot.
'Summary of findings' tables
We assessed the quality of the evidence using the GRADE approach (Schünemann 2013) related to the following main outcomes, which are important for decision‐making:
Participant‐reported scar improvement (long‐term);
Participant‐reported scar improvement (short‐term);
Investigator‐assessed adverse events (short‐term);
Participant‐assessed adverse events (short‐term);
Participant satisfaction;
Quality of life.
We used the GRADEpro Guideline Development Tool (GRADEpro GDT) (GRADEpro GDT 2015) to import data from Review Manager 5 (RevMan 2014) in order to create 'Summary of findings' (SoF) tables. There were many comparisons and consequently several SoF tables. We created SoF tables for the most important comparisons.
We produced a summary of the intervention effect and a measure of quality for each of the above outcomes, using the GRADE approach (Schünemann 2013). This uses five considerations: study limitations, consistency of effect, imprecision, indirectness, and publication bias, to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious, or by two levels for very serious limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, and imprecision of effect estimates or potential publication bias.
Summary of research gaps
We summarised the research uncertainties by mapping the research gaps (Table 9) using a PICOT (population, intervention, comparison, outcome, time) framework (Robinson 2013).
2. Summary of research gaps.
| Gap No. | Reason(s) for Gap* |
POPULATION (P) |
INTERVENTION (I) |
COMPARISON (C) |
OUTCOMES (O) |
SETTING (S) |
Free Text Gap |
| 1 | A1 | People with acne scars | Fractional Laser | No treatment | Participant‐reported scar improvement (long‐term) | Hospital‐based/outpatient | Lack of a validated standardised improvement scale |
| 2 | A1 D4 |
People with acne scars | Fractional Laser | Non‐fractional non‐ablative laser | Participant‐reported scar improvement (long‐term) | Hospital‐based/outpatient | Lack of a validated standardised improvement scale |
| 3 | A2 A3 A4 B2 C1 |
People with acne scars | Fractional Laser | Non‐fractional non‐ablative laser | Participant‐reported scar improvement (short‐term) | Hospital‐based/outpatient | Lack of a validated standardised improvement scale |
| 4 | A1 D4 |
People with acne scars | Fractional Laser | Radiofrequency | Participant‐reported scar improvement (long‐term) | Hospital‐based/outpatient | Lack of a validated standardised improvement scale |
| 5 | A2 A3 B2 C1 |
People with acne scars | Fractional Laser | Radiofrequency | Participant‐reported scar improvement (short‐term) | Hospital‐based/outpatient | Lack of a validated standardised improvement scale |
| 6 | A1 | People with acne scars | Fractional Laser | Chemical peeling | Participant‐reported scar improvement (short‐ and long‐term) | Hospital‐based/outpatient | Lack of a validated standardised improvement scale |
| 7 | A2 A3 B2 C1 |
People with acne scars | Fractional Laser | Combined chemical peeling plus needling | Participant‐reported scar improvement (long‐term) | Hospital‐based/outpatient | Lack of a validated standardised improvement scale |
| 8 | A1 | People with acne scars | Fractional Laser | Combined chemical peeling plus needling | Participant‐reported scar improvement (short‐term) | Hospital‐based/outpatient | Lack of a validated standardised improvement scale |
| 9 | A1 | People with acne scars | Fractional Laser | Microdermabrasion | Participant‐reported scar improvement (short‐ and long‐term) | Hospital‐based/outpatient | Lack of a validated standardised improvement scale |
| 10 | A1 | People with acne scars | Fractional Laser | Needling | Participant‐reported scar improvement (short‐ and long‐term) | Hospital‐based/outpatient | Lack of a validated standardised improvement scale |
| 11 | A1 | People with acne scars | Fractional Laser | Injectible fillers | Participant‐reported scar improvement (short‐ and long‐term) | Hospital‐based/outpatient | Lack of a validated standardised improvement scale |
| 12 | A1 D4 |
People with acne scars | Chemical peeling | Placebo/no treatment | Participant‐reported scar improvement (long‐term and short‐term) | Hospital‐based/outpatient | Lack of a validated standardised improvement scale |
| 13 | A2 A3 B2 C1 |
People with acne scars | Chemical peeling | Combined chemical peeling with any active intervention | Participant‐reported scar improvement (long‐term) | Hospital‐based/outpatient | Lack of a validated standardised improvement scale |
| 14 | A1 | People with acne scars | Chemical peeling | Combined chemical peeling with any active intervention | Participant‐reported scar improvement (short‐term) | Hospital‐based/outpatient | Lack of a validated standardised improvement scale |
| 15 | A1 D4 |
People with acne scars | Chemical peeling | Needling | Participant‐reported scar improvement (long‐term) | Hospital‐based/outpatient | Lack of a validated standardised improvement scale |
| 16 | A2 A3 B2 C1 |
People with acne scars | Chemical peeling | Needling | Participant‐reported scar improvement (short‐term) | Hospital‐based/outpatient | Lack of a validated standardised improvement scale |
| 17 | A1 D4 |
People with acne scars | Injectable fillers | Placebo/no treatment | Participant‐reported scar improvement (long‐term) | Outpatient | Lack of a validated standardised improvement scale |
| 18 | A2 C1 |
People with acne scars | Injectable fillers | Placebo/no treatment | Participant‐reported scar improvement (short‐term) | Outpatient | lack of a validated standardised improvement scale |
| 19 | A1 | People with acne scars | Injectable fillers | Autologous bone marrow stem‐cell transplant | Participant‐reported scar improvement (short‐ and long‐term) | Hospital‐based | Lack of a validated standardised improvement scale |
| 20 | A1 | People with acne scars | Microdermabrasion | No treatment | Participant‐reported scar improvement (short‐ and long‐term) | Outpatient | Lack of a validated standardised improvement scale |
| 21 | A1 | People with acne scars | Microdermabrasion | Needling | Participant‐reported scar improvement (short‐ and long‐term) | Outpatient | Lack of a validated standardised improvement scale |
| 22 | A1 | People with acne scars | Microdermabrasion | Subcision | Participant‐reported scar improvement (short‐ and long‐term) | Outpatient | Lack of a validated standardised improvement scale |
| 23 | A1 | People with acne scars | Needling | No treatment | Participant‐reported scar improvement (short‐ and long‐term) | Outpatient | Lack of a validated standardised improvement scale |
| 24 | A1 | People with acne scars | Needling | Subcision | Participant‐reported scar improvement (short‐ and long‐term) | Outpatient | Lack of a validated standardised improvement scale |
* Reasons for Gap
Insufficient or imprecise information: A1 = No studies; A2 = Limited number of studies; A3 = Sample sizes too small; A4 = Estimate of effect is imprecise Information at 'Risk of bias': B1 = Inappropriate study design; B2 = Major methodological limitations in studies Inconsistency or unknown consistency: C1 = Consistency unknown (only 1 study); C2 = Inconsistent results across studies Not the right information: D1 = Results not applicable to population of interest; D2 = Inadequate duration of interventions/comparisons; D3 = Inadequate duration of follow‐up; D4 = Optimal/most important outcomes not addressed; D5 = Results not applicable to setting of interest
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
As shown in Figure 1, our search identified 288 citations. After removing duplicates, we assessed 216 citations. We excluded 158 citations because the title, abstract, or both did not meet our inclusion criteria. We sought the full text of the remaining 58 citations. We included 24 RCTs and excluded 23 studies. Nine studies are awaiting classification, and two are ongoing studies.
1.
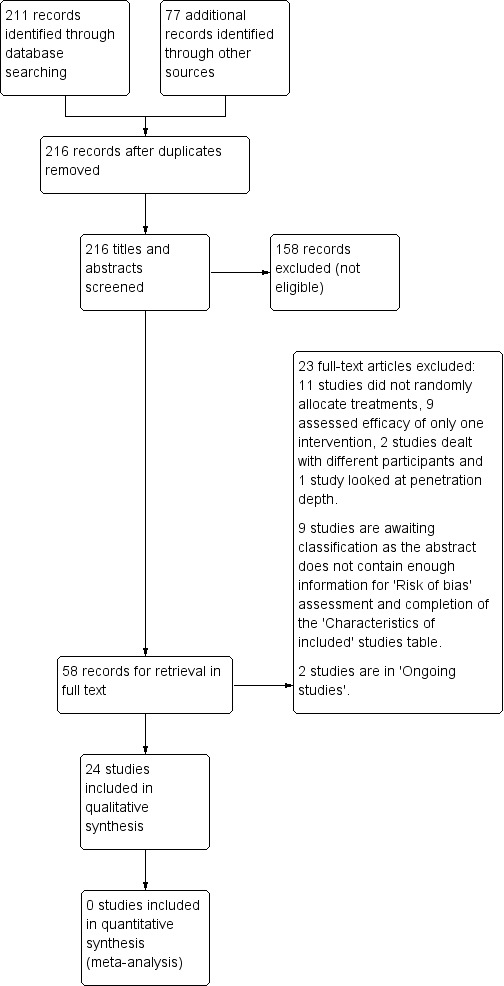
Study flow diagram.
Included studies
This review includes 24 RCTs (published in 27 reports) with a total of 789 participants. Two papers reported on Alam 2014; two papers reported on Leheta 2011 ; and two papers reported on Manuskiatti 2013. See Characteristics of included studies tables for detailed descriptions.
Design
We identified seven parallel RCTs (Asilian 2011; Chae 2015; Erbağci 2000; Karnik 2014; Leheta 2011; Leheta 2014a; Leheta 2014b) and 17 within‐individual (split‐face) RCTs. Seven studies (two parallel RCTs (Erbağci 2000; Karnik 2014) and five split‐face RCTs (Alam 2014; Bernstein 2001; Hedelund 2010; Hedelund 2012; Munavalli 2013)) compared an active intervention to a placebo or no treatments. The remaining 17 RCTs compared active interventions.
Sample sizes
The number of participants in the included studies ranged from 6 to 147. Fifteen of the 24 included trials, (one parallel (Leheta 2014a) and 14 split‐face (Alam 2014; Bernstein 2001; Cho 2010; Hedelund 2010; Hedelund 2012; Kim 2009; Lee 2009; Lee 2011; Linkner 2014; Manuskiatti 2013; Min 2009; Rongsaard 2014; Sage 2011; Tanzi 2004)), had a small sample sizes of fewer than 30 participants.
Setting
Of the 24 included trials, 23 were single‐centre and one study (Karnik 2014) was multicentre. They were conducted in different countries (China 1, Denmark 2, Egypt 3, Iran 2, South Korea 6, Thailand 2, Turkey 1, and USA 7 studies).
Participants
All of the included trials included adults aged 18 years or older. Twenty RCTs (706 participants) enrolled both men and women, three RCTs (75 participants) enrolled only women and one RCT (8 participants) enrolled only men. Nineteen RCTs enrolled 718 individuals with atrophic acne scars and five RCTs enrolled 71 individual with mixed atrophic and hypertrophic acne scars. We did not find any trials that included any information on acne scars on the back.
Interventions
The included trials assessed the effects of interventions for treating facial acne scars including:
Non‐fractional non‐ablative laser
Fractional laser
Fractional radiofrequency
Chemical peeling
Injectable filler
Needling
Subcision
-
Combined interventions
Fractional laser plus intradermal platelet‐rich plasma (PRP)
Fractional laser plus punch elevation
Microdermabrasion plus photodynamic therapy with aminolevulinic acid (ALA‐PDT)
Needling plus chemical peeling
Outcomes
Of the 24 included trials, 19 reported improvement of acne scars, 23 studies reported adverse effects and eight studies reported participant satisfaction. None of the included trials reported quality of life.
Funding source
Of the 24 included trials, five were supported by industry, four by academic institutions; the other 15 trials did not report their funding source.
Studies awaiting classification
We planned in the protocol not to include studies reported as abstracts‐only as we could not extract enough information for 'Risk of bias' assessment and completion of the Characteristics of included studies tables. Please see details of these nine studies under Characteristics of studies awaiting classification.
Ongoing Studies
We identified two ongoing trials ( NCT02216864; NCT02643628). The first ongoing trial (NCT02216864) is a split‐face RCT of multiple subcision versus no treatment. The second ongoing trial (NCT02643628) is a parallel RCT of microneedling versus combined microneedling and injectable filler. We present the details of these trials in the 'Characteristics of ongoing studies' tables.
Excluded studies
We excluded 23 studies. We list the reasons for exclusion in the Characteristics of excluded studies tables. In our criteria for included studies we did not plan to consider the same intervention with different settings such as different treatment levels (e.g. Alexis 2011), different treatment time intervals (Bjørn 2014), different wavelengths (Yaghmai 2005; Yuan 2014), different fluences or power (Jung 2010; Laubach 2009; Srivastava 2009), or different depths of penetration (Tanghetti 2013).
Risk of bias in included studies
We include a plot of the distribution of review authors' judgements across studies for each 'Risk of bias' domain in Figure 2 and a summary of review authors' judgements for each 'Risk of bias' domain for each study in Figure 3. We present further details in the 'Risk of bias' tables in the Characteristics of included studies section. The risk of bias of the included trials varied from low to high.
2.
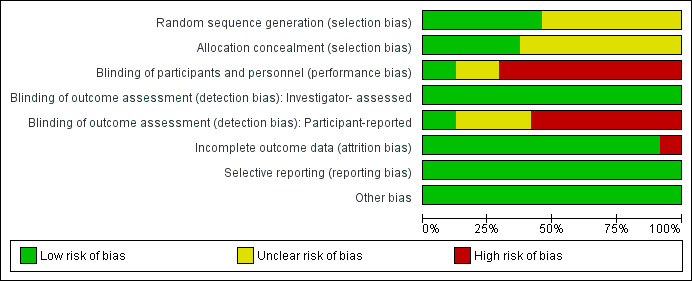
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
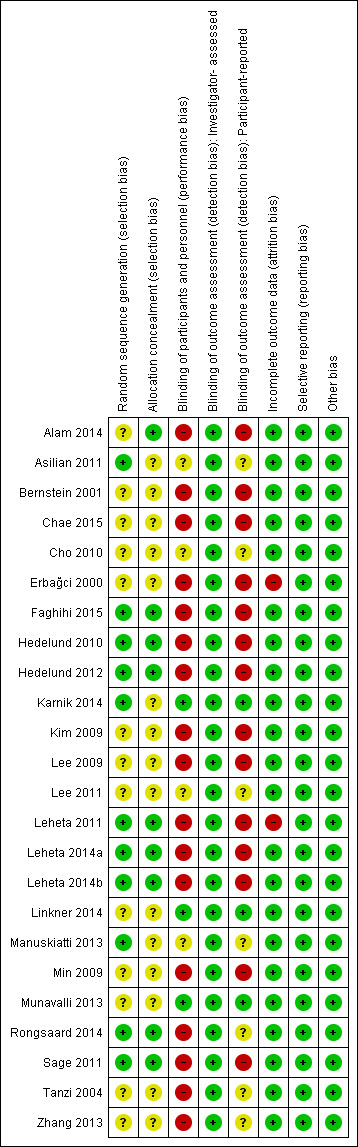
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Eleven trials used an adequate method of randomisation (Asilian 2011; Faghihi 2015; Hedelund 2010; Hedelund 2012; Karnik 2014; Leheta 2011; Leheta 2014a; Leheta 2014b; Manuskiatti 2013; Rongsaard 2014; Sage 2011), while the other 13 trials did not provide sufficient information about the sequence generation process to permit judgement.
Allocation could not be foreseen in nine trials (Alam 2014; Faghihi 2015; Hedelund 2010; Hedelund 2012; Leheta 2011; Leheta 2014a; Leheta 2014b; Rongsaard 2014; Sage 2011), so we judged them to be at low risk of bias, while it was unclear if allocation was concealed in the other 15 trials.
Blinding
Three trials reported blinding of investigators (Karnik 2014; Linkner 2014; Munavalli 2013) and we judged these trials to be at a low risk of performance bias. There was insufficient information about the blinding of investigators in four trials (Asilian 2011; Cho 2010; Lee 2011; Manuskiatti 2013) to permit judgement. The investigators were not blinded In 17 trials (Alam 2014; Bernstein 2001; Chae 2015; Erbağci 2000; Faghihi 2015; Hedelund 2010; Hedelund 2012; Kim 2009; Lee 2009; Leheta 2011; Leheta 2014a; Leheta 2014b; Min 2009; Rongsaard 2014; Sage 2011; Tanzi 2004; Zhang 2013) because the control arm was easily distinguishable from the treatment arm during treatment. We judged these 17 trials to be at high risk of performance bias.
All 24 trials reported that a blinded investigator assessed the outcome. We judged the 24 trials to be at a low risk of detection bias for outcome assessment.
Three trials reported blinding of participants (Karnik 2014; Linkner 2014; Munavalli 2013). There was insufficient information about the blinding of participants in seven trials (Asilian 2011; Cho 2010; Lee 2011; Manuskiatti 2013; Rongsaard 2014; Tanzi 2004; Zhang 2013) to permit judgement. In 14 trials (Alam 2014; Bernstein 2001; Chae 2015; Erbağci 2000; Faghihi 2015; Hedelund 2010; Hedelund 2012; Kim 2009; Lee 2009; Leheta 2011; Leheta 2014a; Leheta 2014b; Min 2009; Sage 2011) participants were not blinded because the control arm was easily distinguishable from the treatment arm. We judged these 14 trials to be at a high risk of detection bias because the outcome assessment was likely to be influenced by lack of blinding of participants.
Incomplete outcome data
The risk of attrition bias was high in two trials (Erbağci 2000; Leheta 2011) because of a high dropout rate. The risk of attrition bias was low in the remaining 22 trials because of a low or null dropout rate
Selective reporting
All trials reported their prespecified outcomes that are of interest in the review in the prespecified way. We judged all 24 trials to be at a low risk of reporting bias.
Other potential sources of bias
We judged the 24 trials to be at a low risk of other potential sources of bias, e.g. baseline imbalance and blocked randomisation in unblinded trials.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Summary of findings for the main comparison. Fractional laser versus non‐fractional non‐ablative laser for acne scars.
| Fractional laser versus non‐fractional non‐ablative laser for acne scars | ||||||
| Patient or population: people with acne scars Settings: hospital‐based Intervention: fractional laser versus non‐fractional non‐ablative laser | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Fractional laser versus non‐fractional non‐ablative laser | |||||
| Participant‐reported scar improvement (long‐term) | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| Participant‐reported scar improvement (short‐term) N of participants with > 50% improvement in acne scars Follow‐up: mean 6 months | 94 per 1000 | 375 per 1000 (117 to 1000) | RR 4 (1.25 to 12.84) | 64 (1 study) | ⊕⊝⊝⊝ very low1,2 | ‐ |
| Investigator‐assessed adverse events (short‐term) Hyperpigmentation N of participants with adverse events Follow‐up: mean 4 weeks | 312 per 1000 | 188 per 1000 (78 to 453) | RR 0.6 (0.25 to 1.45) | 64 (1 study) | ⊕⊝⊝⊝ very low1,3 | ‐ |
| Participant‐assessed adverse events (short‐term) Burning N of participants with adverse events Follow‐up: mean 4 weeks | 1000 per 1000 | 1000 per 1000 (940 to 1000) | RR 1 (0.94 to 1.06) | 64 (1 study) | ⊕⊝⊝⊝ very low1,4 | ‐ |
| Participant satisfaction | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded by one level because of unclear allocation concealment and blinding of participant and personnel. 2Downgraded two levels for very serious imprecision because the optimal information size (OIS) is not met (should be 5600) and the CI is extremely wide. 3Downgraded two levels for very serious imprecision because the optimal information size (OIS) is not met (should be around 1200), very small sample size, and the 95% CI around the estimate of effect includes both no effect and appreciable harm. 4Downgraded two levels for very serious imprecision because the optimal information size (OIS) is not met, very small sample size, and the 95% CI around the estimate of effect includes both no effect and appreciable harm.
Summary of findings 2. Fractional laser versus radiofrequency for acne scars.
| Fractional laser versus radiofrequency for acne scars | ||||||
| Patient or population: people with acne scars Settings: hospital‐based Intervention: fractional laser versus radiofrequency | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Fractional laser versus radiofrequency | |||||
| Participant‐reported scar improvement (long‐term) | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| Participant‐reported scar improvement (short‐term) N of participants with > 50% improvement in acne scars Follow‐up: mean 8 weeks | 450 per 1000 | 351 per 1000 (162 to 756) | RR 0.78 (0.36 to 1.68) | 40 (1 study) | ⊕⊝⊝⊝ very low1,2 | Rongsaard 2014 reported a mean improvement of 2.89 for the fractional laser and 2.74 for the radiofrequency |
| Investigator‐assessed adverse events (short‐term) | See comment | See comment | Not estimable | ‐ | See comment | Unclear whether the reported higher events (erythema, oedema, PIH) with the laser are participant‐ or investigator‐assessed |
| Participant‐assessed adverse events (short‐term) | See comment | See comment | Not estimable | ‐ | See comment | More pain with fractional laser was noticed, Rongsaard 2014 and Zhang 2013 reported higher incidence of positive adverse events with fractional laser |
| Participant satisfaction | See comment | See comment | Not estimable | ‐ | See comment | Zhang 2013 reported that 30/33 and 31/33 of participants were satisfied with laser and radiofrequency respectively with no significant difference |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded by one level because of unclear allocation concealment and high blinding of participant and personnel. 2Downgraded two levels for very serious imprecision because the optimal information size (OIS) is not met (should be around 620) and the 95% CI around the estimate of effect includes both no effect and appreciable benefit.
Summary of findings 3. Fractional laser versus combined chemical peeling plus needling for acne scars.
| Fractional laser versus combined chemical peeling plus needling for acne scars | ||||||
| Patient or population: people with acne scars Settings: hospital‐based Intervention: fractional laser versus combined chemical peeling plus needling | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Fractional laser versus combined chemical peeling plus needling | |||||
| Participant‐reported scar improvement (long‐term) N of participants with > 50% improvement in acne scars Follow‐up: mean 12 months | 692 per 1000 | 692 per 1000 (415 to 1000) | RR 1 (0.6 to 1.67) | 26 (1 study) | ⊕⊝⊝⊝ very low1,2 | ‐ |
| Participant‐reported scar improvement (short‐term) | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| Investigator‐assessed adverse events (short‐term) | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| Participant‐assessed adverse events (short‐term) N of participants with adverse events Follow‐up: mean 4 weeks | 1000 per 1000 | 1000 per 1000 (860 to 1000) | RR 1 (0.86 to 1.16) | 25 (1 study) | ⊕⊝⊝⊝ very low1,3 | ‐ |
| Participant satisfaction | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded by one level for high risk of bias regarding blinding of participants and personnel. 2Downgraded two levels for very serious imprecision because the optimal information size (OIS) is not met (should be around 200), very small sample size, and the 95% CI around the estimate of effect includes both no effect and appreciable benefit. 3Downgraded two levels for very serious imprecision because the optimal information size (OIS) is not met, very small sample size, and the 95% CI around the estimate of effect includes both no effect and appreciable harm.
Summary of findings 4. Chemical peeling versus placebo or no treatment for acne scars.
| Chemical peeling versus placebo or no treatment for acne scars | ||||||
| Patient or population: people with acne scars Settings: out‐patient Intervention: chemical peeling versus placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Chemical peeling versus placebo or no treatment | |||||
| Participant‐reported scar improvement (long‐term) | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| Participant‐reported scar improvement (short‐term) | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| Investigator‐assessed adverse events (short‐term) | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| Participant‐assessed adverse events (short‐term) | See comment | See comment | Not estimable | ‐ | See comment | Burning sensation and deep erythema were reported following frosting in some cases from the chemical peeling |
| Participant satisfaction | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| Serious or severe adverse events N of participants with positive severe adverse events Follow‐up: mean 6 months | 0 per 1000 | 0 per 1000 (0 to 0) | RR 5.45 (0.33 to 90.14) | 58 (1 study) | ⊕⊝⊝⊝ very low1,2 | 7/43 participants experienced serious adverse events with chemical peel but 0/15 in the placebo group |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level for high risk of attrition bias. 2Downgraded two levels for very serious imprecision because the optimal information size (OIS) is far from met, extremely wide CI, due to low occurrence of events in control group and small sample size. 95% CI around the estimate of effect includes both no effect and appreciable harm.
Summary of findings 5. Chemical peeling versus combined chemical peeling plus any active intervention for acne scars.
| Chemical peeling versus combined chemical peeling plus any active intervention for acne scars | ||||||
| Patient or population: people with acne scars Settings: hospital‐based Intervention: chemical peeling versus combined chemical peeling plus any active intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Chemical peeling versus combined chemical peeling plus any active intervention | |||||
| Participant‐reported scar improvement (long‐term) N of participants with > 50% improvement in acne scars Follow‐up: mean 8 months | 800 per 1000 | 992 per 1000 (696 to 1000) | RR 1.24 (0.87 to 1.75) | 20 (1 study) | ⊕⊝⊝⊝ very low1,2 | ‐ |
| Participant‐reported scar improvement (short‐term) | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| Investigator‐assessed adverse events (short‐term) | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| Participant‐assessed adverse events (short‐term) N of participants with adverse events Follow‐up: mean 4 weeks | 1000 per 1000 | 1000 per 1000 (830 to 1000) | RR 1 (0.83 to 1.2) | 20 (1 study) | ⊕⊝⊝⊝ very low1,3 | ‐ |
| Participant satisfaction | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded by one level due to high risk of bias with regard to blinding of participants and personnel. 2Downgraded two levels for very serious imprecision because the optimal information size (OIS) is not met (should be around 100), very small sample size, and the 95% CI around the estimate of effect includes both no effect and appreciable benefit. 3Downgraded two levels for very serious imprecision because the optimal information size (OIS) is not met, very small sample size, and the 95% CI around the estimate of effect includes both no effect and appreciable harm.
Summary of findings 6. Chemical peeling versus needling for acne scars.
| Chemical peeling versus needling for acne scars | ||||||
| Patient or population: people with acne scars Settings: hospital‐based Intervention: chemical peeling versus needling | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Chemical peeling versus needling | |||||
| Participant‐reported scar improvement (long‐term) | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| Participant‐reported scar improvement (short‐term) N of participants with > 50% improvement in acne scars Follow‐up: mean 1 months | 667 per 1000 | 747 per 1000 (460 to 1000) | RR 1.13 (0.69 to 1.83) | 27 (1 study) | ⊕⊝⊝⊝ very low1,2 | ‐ |
| Investigator‐assessed adverse events (short‐term) N of participants with adverse events Follow‐up: mean 4 weeks | Study population | RR 16 (0.99 to 258.36) | 27 (1 study) | ⊕⊕⊝⊝ low3 | 6/12 participants experienced adverse events with chemical peel but 0/15 with needling |
|
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Participant‐assessed adverse events (short‐term) N of participants with positive adverse events Follow‐up: mean 4 weeks | 1000 per 1000 | 1000 per 1000 (870 to 1000) | RR 1 (0.87 to 1.15) | 27 (1 study) | ⊕⊝⊝⊝ very low1,4 | ‐ |
| Patient satisfaction N of satisfied participants Follow‐up: mean 4 weeks | 667 per 1000 | 747 per 1000 (460 to 1000) | RR 1.13 (0.69 to 1.83) | 27 (1 study) | ⊕⊝⊝⊝ very low1,2 | ‐ |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded by one level due to high risk of bias regarding blinding of participants and personnel. 2Downgraded two levels for very serious imprecision because the optimal information size (OIS) is not met (should be around 250), very small sample size, and the 95% CI around the estimate of effect includes both no effect and appreciable benefit. 3Downgraded two levels for very serious imprecision because the optimal information size (OIS) is not met, very small sample size, extremely wide CI, and the 95% CI around the estimate of effect includes both no effect and appreciable harm. 4Downgraded two levels for very serious imprecision because the optimal information size (OIS) is not met, very small sample size, and the 95% CI around the estimate of effect includes both no effect and appreciable harm.
Summary of findings 7. Injectable fillers versus placebo or no treatment for acne scars.
| Injectable fillers versus placebo or no treatment for acne scars | ||||||
| Patient or population: people with acne scars Settings: outpatient Intervention: injectable fillers versus placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Injectable fillers versus placebo or no treatment | |||||
| Participant‐assessed scar improvement (long‐term) | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| Participant‐reported scar improvement (short‐term) N of participants with > 50% improvement in acne scars Follow‐up: mean 6 months | 420 per 1000 | 773 per 1000 (550 to 1000) | RR 1.84 (1.31 to 2.59) | 147 (1 study) | ⊕⊕⊕⊝ moderate1 | Munavalli 2013 reported 43% vs 18% improvement with the dermal filler and placebo respectively |
| Participant satisfaction N of satisfied participants Follow‐up: mean 6 months | 520 per 1000 | 848 per 1000 (640 to 1000) | RR 1.63 (1.23 to 2.15) | 147 (1 study) | ⊕⊕⊕⊝ moderate1 | ‐ |
| Participant‐reported adverse events (short‐term) N of participants with positive adverse events Follow‐up: mean 4 weeks | 20 per 1000 | 21 per 1000 (2 to 222) | RR 1.03 (0.1 to 11.1) | 147 (1 study) | ⊕⊕⊝⊝ low2 | ‐ |
| Investigator‐assessed adverse events (short‐term) N of participants with positive adverse events Follow‐up: mean 4 weeks | 260 per 1000 | 174 per 1000 (94 to 330) | RR 0.67 (0.36 to 1.27) | 147 (1 study) | ⊕⊕⊝⊝ low3 | Munavalli 2013 reported comparable incidence of events with dermal filler and placebo |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level for imprecision because the optimal information size (OIS) is not met (should be around 300), although the sample size is not that small. 2Downgraded two levels for very serious imprecision because the optimal information size (OIS) is not met (should be around 1500), small sample size, and the 95% CI around the estimate of effect includes both no effect and appreciable harm. 3Downgraded two levels for very serious imprecision because the optimal information size (OIS) is not met, and the 95% CI around the estimate of effect includes both no effect and appreciable harm.
We reported our prespecified outcomes under the following 14 pair‐wise comparisons:
Non‐fractional non‐ablative laser versus placebo or no treatment
Fractional laser versus non‐fractional non‐ablative laser
Fractional laser versus placebo or no treatment
Fractional laser versus radiofrequency
Fractional laser versus combined fractional laser plus any active intervention
Fractional laser versus chemical peeling
Fractional laser versus combined chemical peeling plus needling
Chemical peeling versus placebo or no treatment
Chemical peeling versus combined chemical peeling plus any active intervention
Chemical peeling versus needling
Needling versus placebo or no treatment
Injectable fillers versus placebo or no treatment
Injectable fillers versus subcision
Combined microdermabrasion plus ALA‐PDT versus combined microdermabrasion plus placebo‐PDT
As we specified in our protocol, we assessed our primary outcome of scar improvement over a time frame of up to 24 weeks (short‐term) and more than 24 weeks (long‐term) and present these outcomes separately as 'Number of participants with > 50% improvement'. We also used this timing for our secondary outcome 'Investigator‐assessed scar improvement' which we also present as 'Number of participants with > 50% improvement'. Our secondary outcome 'Participant satisfaction' was expressed as 'Number of satisfied participants'.
We assessed our adverse events outcomes in the short term up to four weeks after the procedure and in the long term more than four weeks after the procedure.
We employed GRADE methodology to provide an assessment of the quality of the evidence for each of the primary and secondary outcomes.
Nine studies provided no usable data on many outcomes and did not contribute further to the results of this review. For the other studies the main reasons why data could not be used were that our outcomes were not addressed, outcomes were only described and not reported numerically, or insufficient data were available to determine, e.g. standard deviations.
1. Non‐fractional non‐ablative laser versus placebo or no treatments
This analysis includes one within‐individual study (Bernstein 2001; n = 11) in which one cheek was randomised to receive a frequency‐doubled 532 nm Nd:YAG laser for an average of three treatments and the other cheek was kept untreated. This study did not assess our second primary outcome or any of our secondary outcomes. See Analysis 1.1 for a precis of our findings.
1.1. Analysis.
Comparison 1 Non‐fractional non‐ablative laser versus placebo or no treatment, Outcome 1 Within‐individual studies.
| Within‐individual studies | |||
|---|---|---|---|
| Study | Interventions | Summary Outcomes | Comment |
| Bernstein 2001 | One side: Non‐fractional non‐ablative laser (Nd:YAG) Other side: untreated control |
Participant‐reported scar improvement (short‐term): In the treatment arm: average 53.6% (range 10% ‐ 90%) Participant‐reported adverse events (short‐term): No side effects were noted. |
Bernstein 2001 did not assess the primary outcome ‘Serious adverse effects’ and the secondary outcomes ‘Investigator‐assessed scar improvement’, ‘Participant satisfaction’, ‘Quality of life’, ‘Investigator‐assessed adverse events’ and ‘Post‐procedure down time’ |
Primary outcomes
Participant‐reported scar improvement (short‐term): Acne scarring was rated as improved by an average of 53.6%, with a range from 10% to 90%. Eight of 11 participants showed more than 50% improvement in acne scars on the laser‐treated side; there were no data available for the untreated side. We judged this study to be at high risk of detection bias.
2. Fractional laser versus non‐fractional non‐ablative laser
This analysis includes one parallel‐group study (Asilian 2011) in which 64 participants with atrophic acne scars were randomised to receive four sessions of fractional CO₂ laser (four‐week intervals) or the comparator of four sessions of non‐fractional non‐ablative Q Switched 1064 nm Nd:YAG laser (four‐week intervals). This study only assessed three of our secondary outcomes. See Table 1 for our grading of the evidence.
Primary outcomes
Participant‐reported scar improvement (short‐term): At six months post‐treatment 12/32 participants reported more than 50% improvement in acne scarring in the fractional laser arm and 3/32 reported more than 50% improvement in acne scarring in the non‐fractional non‐ablative arm. There was a statistically significant difference in favour of fractional laser (RR 4.00, 95% CI 1.25 to 12.84; very low‐quality evidence; Analysis 2.1). We rated this study at unclear risk of detection bias.
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: All participants (n = 64) completed the allocated treatments, indicating that no‐one experienced adverse effects severe enough to withdraw from the study.
2.1. Analysis.
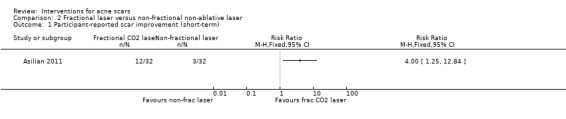
Comparison 2 Fractional laser versus non‐fractional non‐ablative laser, Outcome 1 Participant‐reported scar improvement (short‐term).
Secondary outcomes
Investigator‐assessed scar improvement (short‐term): After treatment, 12/32 participants post‐fractional laser treatment versus 4/32 participants post‐non‐fractional treatment non‐ablative laser showed more than 50% improvement in acne scarring. There was a statistically significant difference in favour of fractional laser (RR 3.00, 95% CI 1.08 to 8.32; Analysis 2.2).
Participant‐reported adverse events (short‐term): All participants in the non‐fractional non‐ablative group (n = 32) reported transient post‐treatment burning sensation but it was more severe in those treated with fractional CO₂ laser (n = 32). The burning sensation lasted for more than a few hours (RR 1.00, 95% CI 0.94 to 1.06; very low‐quality evidence; Analysis 2.3).
Investigator‐assessed adverse events (short‐term): Post‐inflammatory hyperpigmentation lasting for two to three weeks was reported by 6/32 and 10/32 participants treated with non‐fractional non‐ablative laser and fractional CO₂ laser respectively (RR 0.60, 95% CI 0.25 to 1.45; low‐quality evidence Analysis 2.4).
2.2. Analysis.
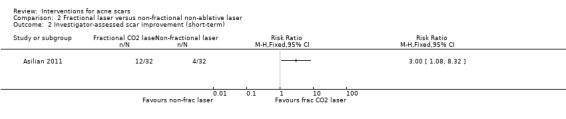
Comparison 2 Fractional laser versus non‐fractional non‐ablative laser, Outcome 2 Investigator‐assessed scar improvement (short‐term).
2.3. Analysis.
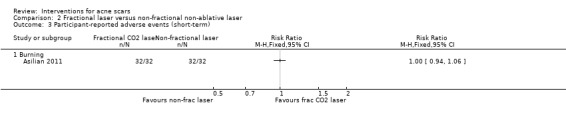
Comparison 2 Fractional laser versus non‐fractional non‐ablative laser, Outcome 3 Participant‐reported adverse events (short‐term).
2.4. Analysis.
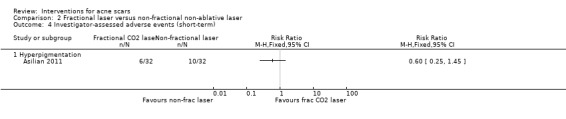
Comparison 2 Fractional laser versus non‐fractional non‐ablative laser, Outcome 4 Investigator‐assessed adverse events (short‐term).
3. Fractional laser versus placebo or no treatment
This analysis includes two within‐individual studies (Hedelund 2010; Hedelund 2012). See Analysis 3.1 for a precis of our findings.
3.1. Analysis.
Comparison 3 Fractional laser versus placebo or no treatment, Outcome 1 Within‐individual studies.
| Within‐individual studies | |||
|---|---|---|---|
| Study | Interventions | Summary Outcomes | Comment |
| Hedelund 2010 | One side: Fractional non‐ablative 1,540‐nm laser Other side: untreated control |
1. Investigator‐assessed improvement in scar texture (short‐term): median (IQR). In the treated arm: 4.5 (2.5 – 6.5); In the untreated arm: 6.0 (4.5 – 8.0) (P = 0.032) 2. Participant satisfaction: Satisfaction scores In the treated arm: median 5.5, IQR 1 – 7 (P = 0.1875) 3. Participant‐reported overall acne scar appearance (short‐term): In the treated arm: number of participants evaluated significantly (1/10), moderately (4/10) or slightly (3/10) improved. 2 participants evaluated the appearance of their scars as no improvement after treatment. 4. Participant‐reported adverse events (short‐term): In the treated arm: Participants experienced moderate pain (median 4.5, IQR 3 – 6.5, P = 0.8302), transient erythema (10/10 participants, P = 0.6013), oedema (7/10 participants, P = 0.3675), superficial crusts (3/10 participants, P = 0.6013) and minor bullae (1/10 participants, P = 1). In the untreated arm: No adverse effects were seen in untreated control areas. |
Hedelund 2010 did not assess the secondary outcome ‘Quality of life’ |
| Hedelund 2012 | One side: Fractional ablative CO₂ laser Other side: untreated control |
1. Investigator‐assessed improvement of scar texture and atrophy (short‐term): Mean values of the 3 evaluators assessment scores: In the treated arm: 3.89 ± 1.74 In the untreated arm: 5.22 ± 2.06, (P < 0.0001) Scar atrophy: In the treated arm: 3.56 ± 1.76 In the untreated arm: 4.89 ± 1.94, (P < 0.0001) 2. Participant satisfaction: Satisfaction scores: In the treated arm: median 4.5, IQR 2 – 7, (P = 0.117) 3. Participant self‐assessments of scar texture improvement (short‐term): median (IQR) In the treated arm: 3 (2 – 6) (P = 0.629) 4. Participant‐reported adverse events (short‐term): In the treated arm: Participants experienced mild to moderate pain (median (IQR); 2 (2 – 4), P = 0.086). Participants responded with erythema (no erythma: 4/8, mild: 8/13; moderate 1/13) and wounds (mild 12/13; moderate 1/13) 2 – 3 days. In the untreated arm: No adverse effects were seen in untreated control areas. 5. Investigator‐assessed adverse events (short‐term): 9/13 participants responded with mild to moderate erythema while all participants responded with mild to moderate wound formation 2 ‐ 3 days post‐treatment. No significant differences were found in skin redness and pigmentation between treated and untreated areas. |
Hedelund 2012 did not assess the primary outcome ‘Serious adverse effects’ and the secondary outcomes ‘Quality of life’. |
Hedelund 2010 was a within‐individual trial with 10 participants in which one area on the site (A) was randomised to receive fractional 1540‐nm Er:Glass laser treatment for three sessions at four‐week intervals and the area on the contralateral site (B) received no treatment. This study did not assess our secondary outcome ‘Quality of life’.
Primary outcomes
Participant‐reported scar improvement (short‐term): The overall acne scar appearance was evaluated by the participants after 12 weeks on a five‐point scale (worse, not improved, slightly improved, moderately improved, significantly improved) and revealed that 8/10 were improved on the laser‐treated side; there were no data available for the untreated side. We judged this study to be at high risk of detection bias.
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: All 10 participants completed the allocated treatments, indicating that no‐one experienced adverse effects severe enough to withdraw from the study.
Secondary outcomes
Investigator‐assessed scar improvement (short‐term): After treatment, scar texture severity score remained constant in untreated areas but improved in treated areas, with an average improvement of 1.5 on the 0 – 10 scale (0 even skin texture without scarring, 5 moderate scarring, and 10 worst possible scarring).
Participant satisfaction: Participants were satisfied with the treatment side with median satisfaction score of 5.5 (interquartile range (IQR) 1 – 7) at week 12 based on a patient satisfaction scale (from score 0, no satisfaction, to 10, best imaginable satisfaction); there were no data available for the untreated side.
Participant‐reported adverse events (short‐term): 10/10 participants reported immediate pain and transient erythema post‐treatment; no adverse effects were seen in untreated control areas.
Investigator‐assessed adverse events (long‐term): No significant differences were found in skin redness and pigmentation between treated and untreated areas (P value not reported).
Post‐procedure down time (days): The skin reactions developed post‐procedure did not influence the participants’ daily activities.
Hedelund 2012 was a within‐individual trial with 13 participants in which one area on the site (A) was randomised to receive fractional CO₂ laser treatment for three sessions at four‐ to five‐week intervals and the area on the contralateral site (B) received no treatment. It did not assess our second primary outcome ‘Serious adverse effects’ or several of our secondary outcomes. One participant left the trial before the end assessment and was not included in the analysis 6 months postoperatively (Hedelund 2012); no mention was made of the cause for withdrawal.
Primary outcomes
Participant‐reported scar improvement (short‐term): Participants (n = 12) reported mild to moderate improvement in scar texture at six months postoperatively on the laser‐treated side, based on a numerical scale from 0 (even skin texture without scarring) to 10 (worst possible scarring); there were no data available for the untreated site. We judged this study to be at high risk of detection bias.
Secondary outcomes
Investigator‐assessed scar improvement (short‐term): Fractional CO₂ laser resurfacing was shown to significantly improve skin texture appearance and atrophy (depth of scars) in 12/12 participants with atrophic acne scars, from a mean of 6.15 (standard deviation (SD) 1.23) for skin texture and 5.72 (SD 1.45) for depth of scars before treatment to a mean of 3.89 (SD 1.74) for skin texture and 3.56 (SD 1.76) for depth of scars at six months (P < 0.0001). These measurements were based on three physicians who were blinded to the assessments and who used a grading scale (0 = even skin texture without scarring or atrophy to 10 = worst possible scarring or atrophy) (P < 0.0001).
Participant satisfaction: All participants (n = 13) were satisfied with the treatment, with a median satisfaction score of 4.5 (IQR 2 – 7) at week 24 based on a numerical scale from 0 (unsatisfied) to 10 (maximal satisfaction); there were no data available for the untreated side.
Participant‐reported adverse events (short‐term): Participants (n = 13) experienced mild to moderate pain on the treated side. No adverse effects were seen in untreated control areas.
Investigator‐assessed adverse events (short‐term): 9/13 participants responded with mild to moderate erythema while all participants responded with mild to moderate wound formation two to three days post‐treatment. No significant differences were found in skin redness and pigmentation between treated and untreated areas.
Post‐procedure down time (days): The skin reactions developed post‐procedure did not influence the participants’ daily activities.
4. Fractional laser versus radiofrequency
This analysis includes one parallel‐group study (Chae 2015) and two within‐individual studies (Rongsaard 2014; Zhang 2013). See Table 2 for our grading of the evidence.
Chae 2015 was a parallel trial in which 40 participants were randomly divided into two equal groups to receive three sessions (four‐week intervals) of 1550 nm Er:Glass fractional laser or the comparator of three sessions (four‐week intervals) with the fractional radiofrequency device. This study did not assess several of our secondary outcomes.
Primary outcomes
Participant‐reported scar improvement (short‐term): 7/20 participants reported more than 50% improvement in the appearance of acne scars in the fractional laser arm, while in the radiofrequency microneedle arm 9/20 participants reported more than 50% acne scar improvement using participant’s self assessment of percentage of improvement. No statistically significant difference was reported (RR 0.78, 95% CI 0.36 to 1.68; very low‐quality evidence; Analysis 4.1). We rated this study at high risk of detection bias.
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: No serious adverse effects were reported. All 40 participants completed the trial as planned.
4.1. Analysis.
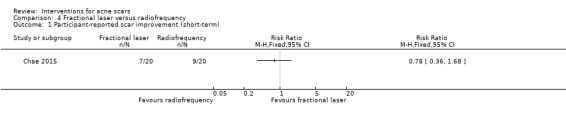
Comparison 4 Fractional laser versus radiofrequency, Outcome 1 Participant‐reported scar improvement (short‐term).
Secondary outcomes
Investigator‐assessed scar improvement (short‐term): At eight weeks post‐treatment, two blinded physicians who were not involved in the trial assessed scar improvement in both arms using the physician’s global assessment scale. Eleven of 20 participants in the fractional laser group and 8/20 participants in the radiofrequency microneedle group showed more than 50% improvement following treatment, but there was no statistically significant difference between them (RR 1.38, 95% CI 0.71 to 2.68; Analysis 4.2). The mean Clinical Evaluation Scale for Acne Scarring (échelle d’évaluation clinique des cicatrices d’acné (ECCA)) grading scale of both groups decreased significantly over the 20‐week time period of the study. In the fractional laser group, the mean ECCA grading scale was reduced from 74.25 to 55.5 (P < 0.001), with a 25.0% reduction from baseline scale. In the radiofrequency group, the mean ECCA grading scale decreased from 68.75 to 56.0 (P < 0.01), with an 18.6% reduction. At the end of the study, in both groups, there were meaningful decreases from the baseline ECCA grade. However, the difference between both interventions was not statistically significant (P > 0.05).
Participant‐reported adverse events (short‐term): Overall, participants experienced more pain with the fractional laser than with the radiofrequency device, with a mean of 5.5 (SD 1.10) and 4.7 (SD 1.08) respectively on visual analogue scale (VAS) (P value not reported).
Investigator‐assessed adverse events (short‐term): 5/12 and 3/12 participants experienced erythema for more than five days with the fractional laser and radiofrequency microneedle respectively. Oedema over more than five days was reported in 3/20 and 1/20 participants with the fractional laser and the radiofrequency microneedle respectively. Post‐inflammatory hyperpigmentation was reported in 2/20 participants with the fractional laser only, while none was found with the radiofrequency device (unclear whether they are participant‐ or investigator‐reported) (P value not reported).
4.2. Analysis.
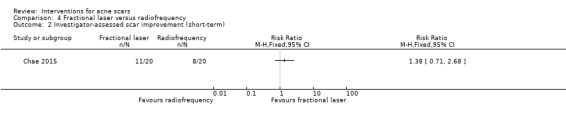
Comparison 4 Fractional laser versus radiofrequency, Outcome 2 Investigator‐assessed scar improvement (short‐term).
With regard to the two within‐individual studies see Analysis 4.3 for a precis of our findings.
4.3. Analysis.
Comparison 4 Fractional laser versus radiofrequency, Outcome 3 Within‐individual studies.
| Within‐individual studies | |||
|---|---|---|---|
| Study | Interventions | Summary Outcomes | Comment |
| Rongsaard 2014 | One side: Fractional non‐ablative 1,550‐nm Er:Glass laser Other side: Fractional radiofrequency device |
1. Participant‐reported scar improvement (short‐term): Mean improvement grade of participants was 2.74 ± 0.73 for the fractional RF device and 2.89 ± 0.57 for the fractional laser. 2. Investigator‐assessed scar improvement (short‐term): Mean improvement grade in acne scars was 2.70 ± 0.37 for the fractional RF device and 2.86 ± 0.42 for the fractional laser. 3. Improvement in facial texture: There were statistically significant (P < .001) reductions in texture scores after treatment with the fractional RF (2.71 ± 1.92) and the fractional laser (2.94 ± 1.84) 4. Participant satisfaction: In fractional RF arm: 6 participants (31.6%) rated themselves as moderately satisfied, 10 (52.6%) rated as very satisfied, and three (15.8%) rated as most satisfied. In fractional laser arm: 5 participants (26.3%) rated themselves as moderately satisfied, 13 (68.4%) rated as very satisfied, and one (5.3%) rated as most satisfied. 5. Patient‐reported adverse events (short‐term): In fractional RF arm: Mean pain scores were 5.90 ± 1.21, duration of facial erythema was 3.10 ± 1.17 days, duration of scab shedding was 5.00 ± 2.60 days, duration of facial dryness was 3.85 ± 3.15 days In fractional laser arm: Mean pain scores were 7.75 ± 1.37, duration of facial erythema was 2.90 ± 1.65 days, duration of scab shedding was 3.45 ± 2.95 days, duration of facial dryness was 3.25 ± 2.71 days. |
Rongsaard 2014 did not assess the secondary outcomes ‘Quality of life’ ‘Investigator‐reported adverse events’ and ‘Post‐procedure down time’. |
| Zhang 2013 | One side: Fractional ablative CO₂ (FS) laser Other side: fractional radiofrequency device (RF) |
1. Investigator‐assessed scar improvement (short‐term): In fractional RF arm: ECCA scores fell from 51.1 ± 14.2 to 22.3 ± 8.6, with 56.4% improvement. In CO₂ FS arm: ECCA scores fell from 48.8 ± 15.1 to 19.9 ± 7.9, with 59.2% improvement. 2. Participant satisfaction: In fractional RF arm: 22 (66.7%) were very satisfied or satisfied, 9 (27.3%) were slightly satisfied, and 2 (6.0%) were unsatisfied. In CO₂ FS arm: 20 (60.6%) were very satisfied or satisfied, 10 (30.3%) were slightly satisfied, and 3 (9.1%) were unsatisfied. 3. Participant‐reported adverse events (short‐term): In fractional RF arm: Mean duration of post‐therapy erythema and scaling was 5.7 days, no PIH was observed in this arm. In CO₂ FS arm: Mean duration of post‐therapy erythema and scaling was 10.2 days, 12 participants (36.4%) experienced post‐inflammatory hyperpigmentation (PIH) after 30 of 99 treatment sessions (30.3%). |
Zhang 2013 did not assess the primary outcome ‘Participant‐reported scar improvement’ and the secondary outcomes ‘Quality of life’ ‘Investigator‐reported adverse events’ and ‘Post‐procedure down time’. |
Rongsaard 2014 was a within‐individual trial in which one side of the face was randomised to be treated with the fractional 1550‐nm Er:Glass laser and the other side of the face treated using the fractional bipolar radiofrequency (RF) device for three treatment sessions at four‐week intervals. This study did not assess several of our secondary outcomes .
Primary outcomes
Participant‐reported scar improvement (short‐term): Mean improvement grade in acne scars after treatment was 2.89 (SD 0.57) for the fractional laser Er:Glass (n = 19) and 2.74 (SD 0.73) for the fractional bipolar RF (n = 19) devices respectively, using the grading scale: 0 = no improvement; 1 = < 25% (mild) improvement; 2 = 25% – 50% (moderate) improvement; 3 = 51% – 75% (good) improvement; 4 = > 75% (excellent) improvement), with no significant difference between the treated sides (P value not reported). This study showed unclear risk of detection bias.
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: 1/20 withdrew from the study because of prolonged dyspigmentation which negatively affected his quality of life.
Secondary outcomes
Investigator‐assessed scar improvement (short‐term): The mean improvement grade in acne scars after treatment was 2.86 (SD 0.42) and 2.70 (SD 0.37) for the fractional Er:Glass laser (n = 19) and the fractional bipolar RF (n = 19) devices respectively, with no significant difference between the treated sides (P value not mentioned).
Participant satisfaction: All participants (n = 19) were satisfied with both treatments.
Participant‐reported adverse events (short‐term): Pain, transitory facial erythema, facial dryness, and scab construction had been reported for both interventions. The pain score reported with the fractional laser was higher than with the fractional RF (mean difference = 1.85 (SD 1.30); P < 0.001), while the length of scab‐shedding treatment was longer with the fractional RF than with the fractional laser (Rongsaard 2014) (mean difference = 1.55 (SD 2.65) days; P = 0.01). There was no significant difference between the two devices regarding duration of facial erythema and dryness (P = 0.60 and 0.10 respectively).
Zhang 2013 was a within‐individual trial of 33 participants in which one facial half was randomised to receive treatment with a 10,600‐nm CO₂ fractional laser (FS) and the other half received treatment with a fractional microplasma radiofrequency device (RF) for three treatment sessions at six‐ to 12‐week (average eight‐week) intervals. This study did not assess our first primary outcome or several of our secondary outcomes .
Primary outcomes
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: All participants completed the allocated treatments and none withdrew from the study.
Secondary outcomes
Investigator‐assessed scar improvement (short‐term): After treatment, acne scars improved in all participants (n = 33) by 59.2% and 56.4% following CO₂ FS and fractional RF treatments respectively using the ECCA score, with no significant difference (P = 0.93).
Participant satisfaction: After treatment, 90.9% (30/33) and 93.9% (31/33) of participants were satisfied with the CO₂ FS and fractional RF treatments respectively (RR 1.5) with no significant difference (P = 0.16).
Participant‐reported adverse events (short‐term): Post‐therapy erythema and scaling remained for longer on the CO₂ FS side than on the fractional RF side, with mean duration of 10.2 days and 5.7 days respectively (P < 0.001).
Investigator‐assessed adverse events (long‐term): 12/33 (36.4%) experienced post‐inflammatory hyperpigmentation with 45.8 days average duration on the CO₂ FS side; no post‐inflammatory hyperpigmentation was observed on the fractional RF sides (P < 0.001).
5. Fractional laser versus combined fractional laser plus any active intervention
This comparison includes two within‐individual studies (Faghihi 2015; Lee 2011). See Analysis 5.1.for a precis of our findings.
5.1. Analysis.
Comparison 5 Fractional laser versus combined fractional laser plus any active intervention, Outcome 1 Within‐individual studies.
| Within‐individual studies | |||
|---|---|---|---|
| Study | Interventions | Summary Outcomes | Comment |
| Faghihi 2015 | One side: Fractional ablative CO₂ laser Other side: Fractional CO₂ laser plus punch elevation . |
Serious adverse effects: All participants completed the allocated treatments and were included in the analyses. Investigator‐assessed scar improvement (short‐term: 4 months): 26/42 participants post‐fractional laser alone versus 31/42 participants post‐combined fractional laser and punch elevation reported > 50% improvement. Participant satisfaction: Participants were more satisfied with the combined fractional laser with punch elevation treatment than with the fractional laser alone. Participant‐reported adverse events (< 4 weeks): The most commonly reported adverse effect was transient erythema and crusting lasting for an average of 3 – 4 and 4 – 7 days, respectively. Investigator‐assessed adverse events (long‐term) (> 4 weeks): Mild postinflammatory hyperpigmentation was observed in 21.4% of participants which lasted < 6 months. |
Faghihi 2015 did not assess the primary outcome ‘Participant‐reported scar improvement’ and the secondary outcomes ‘Quality of life’ and ‘Post‐procedure down time’. |
| Lee 2011 | One side: Fractional ablative CO₂ laser + intradermal injection with normal saline Other side: Fractional ablative CO₂ laser + intradermal treatment with autologous PRP. |
Serious adverse effects: All participants completed the allocated treatments and were included in the analysis. Investigator‐assessed scar improvement (4 months): the mean overall degree of clinical improvement was 2.3 (SD 0.5) on the fractional laser alone side and 2.7 (SD 0.7) on the combined fractional laser plus PRP side (P = 0.03). Participant‐reported adverse events (< 4 weeks): All participants were observed to experience some degree of post‐treatment crusting. Oedema lasted an average of 7.1 (SD 1.5) days on the control side and 6.1 (SD 1.1) days on the experimental side (P = 0.04). Investigator‐assessed adverse events (3 months): erythema on the combined fractional laser + PRP side was significantly less and improved faster than on the fractional laser alone side. Post‐treatment oedema on the combined fractional laser + PRP side also improved faster than the fractional laser alone side. |
Lee 2011 did not assess the primary outcomes ‘Participant‐reported failure of treatment’, ‘Participant‐reported scar improvement’ nor the secondary outcomes ‘Investigator‐reported failure of treatment’ ‘Participant satisfaction', ‘Quality of life’ and ‘Post‐procedure down time’. |
Faghihi 2015 was a within‐individual trial of 42 participants in which one side of the participant's face was randomised to be treated using the 10,600nm fractional CO₂ laser alone for two sessions with a one‐month interval and the other side of the face was treated with the same fractional CO₂ laser plus one session of punch elevation before the laser sessions. This study did not assess our first primary outcome or several of our secondary outcomes.
Primary outcomes
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: All participants (n = 42) completed the allocated treatments and were included in the analyses.
Secondary outcomes
Investigator‐assessed scar improvement (short‐term): After treatment, 26/42 participants post‐treatment with the fractional laser alone versus 31/42 participants post‐treatment with the combined fractional laser and punch elevation, reported more than a 50% improvement in their acne scars (RR 1.45) using the following grading scale (1 ≤ 25% (minimal) improvement; 2 = 5% – 50% (moderate) improvement; 3 = 51% ‐ 75% (good) improvement; 4 ≥ 75% (excellent) improvement). Fractional CO₂ laser treatment combined with punch elevation produced better improvement in acne scars than fractional CO₂ laser treatment alone (P = 0.02).
Participant satisfaction: Participants were more satisfied with the combined fractional laser with punch elevation treatment than with the fractional laser alone; mean acne scar improvement was 7.8 (SD 1.6) and 6.8 (SD 1.9) respectively using a VAS; 0 as no satisfaction, and 10 as the best possible satisfaction) (P = 0.009).
Participant‐reported adverse events (short‐term): Transient erythema for three to four days and crusting lasting for about four to seven days were the most frequently testified adverse events. Transitory burning and redness after treatment were seen in all participants (n = 42) on both treatment sides which resolved without any treatment.
Investigator‐assessed adverse events (long‐term): Mild postinflammatory hyperpigmentation was observed in 9/42 (21.4%) of participants one month after treatment, lasting for less than six months.
Lee 2011 was a within‐individual trial of 14 participants in which the entire face was treated with a fractional ablative CO₂ laser, and then one facial half was randomised to receive an intradermal injection with 0.3 ml normal saline and the other half received an intradermal treatment with 0.3 ml autologous platelet‐rich plasma (PRP). One month later, another similar treatment session was given to all participants. This study did not assess our first primary outcome or several of our secondary outcomes.
Primary outcomes
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: All participants (n = 14) completed the allocated treatments and were included in the analysis.
Secondary outcomes
Investigator‐assessed scar improvement (short‐term): Four months after the final treatment, the mean overall degree of clinical improvement was 2.3 (SD 0.5) on the fractional laser‐only side and 2.7 (SD 0.7) on the combined fractional laser plus PRP side, with better results on the combined fractional laser plus PRP side (P = 0.03) using a quartile grading scale (0 = no improvement; 1 = < 25% improvement; 2 = 25% – 50% improvement; 3 = 51% – 75% improvement; 4 = > 75% improvement).
Participant‐reported adverse events (short‐term): All participants (n = 14) reported some degree of post‐treatment crusting, which lasted longer on the fractional laser alone side for an average of 6.8 (SD 1.0) days compared to the combined fractional laser plus PRP side with an average of 5.9 (SD 1.1) days (P = 0.04). Oedema also lasted longer on the fractional laser alone side (n = 14) for an average of 7.1 (SD 1.5) days than on the combined fractional laser plus PRP side (n = 14) with an average of 6.1 (SD 1.1) days (P = 0.04).
Investigator‐assessed adverse events (short‐term): The resurfacing‐associated erythema on the combined fractional laser plus PRP side was significantly less and improved faster than on the fractional laser alone side at post‐treatment day four (P = 0.047). Post‐treatment oedema on the combined fractional laser plus PRP side also improved faster than the fractional laser alone side, although the difference was not statistically significant (P value not mentioned). None of the other adverse events (petechiae, oozing, dyschromia, infection, scarring, or blistering) occurred in any participant.
6. Fractional laser versus chemical peeling
One within‐individual study (Kim 2009) of 20 participants addressed this comparison, in which one side of the face was randomised to be treated with the 1550 nm Er:Glass fractional laser for three sessions (six‐week intervals) and the other side was treated with the chemical reconstruction of skin scars (CROSS) chemical peeling method twice every 12 weeks. This study did not assess our first primary outcome or several of our secondary outcomes. See Analysis 6.1 for a precis of our findings.
6.1. Analysis.
Comparison 6 Fractional laser versus chemical peeling, Outcome 1 Within‐individual studies.
| Within‐individual studies | |||
|---|---|---|---|
| Study | Interventions | Summary Outcomes | Comment |
| Kim 2009 | One side: Fractional non‐ablative 1,550 nm Er:Glass laser Other side: Chemical peeling |
Serious adverse effects: 18/20 participants completed the trial and were included in the data. 1 participant dropped out because of slight discomfort of the treatment such as pain and erythema, and the other participant dropped out because of scheduling conflicts. Investigator‐assessed scar improvement (12 weeks): The overall average improvement grades by dermatologists were 2.51 in the fractional laser site and 2.44 in the chemical peeling site. Participant‐reported adverse events: Pain was noted 4.49 in the laser sides and 3.33 in the chemical peeling sides. Mean erythema lasting days were noted as 3.30 days in the laser sides and 12.13 days in the chemical peeling sides. Post‐procedure down time: Mean downtimes were noted as 3.17 days in the laser sides and 9.72 days in the chemical peeling sides. |
Kim 2009 did not assess the primary outcomes 'Participant‐reported failure of treatment’, ‘Participant‐reported scar improvement’ and the secondary outcomes ‘Investigator‐reported failure of treatment’, ‘Participant satisfaction’, ‘Quality of life’ and ‘Investigator‐assessed adverse events’. |
Primary outcomes
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: 18/20 participants finished the trial and were included in the data. One participant left the trial because of minor discomfort with the treatment from pain and redness, and the other left the trial because of timetabling clashes (Kim 2009).
Secondary outcomes
Investigator‐assessed scar improvement (short‐term): The overall average improvement grades were 2.51 in the fractional laser site (n = 18) and 2.44 in the chemical peeling site (n = 18) using a quartile scale (0 = no improvement; 1 = 1% – 25% improvement; 2 = 26% – 50% improvement; 3 = 51%– 75% improvement; 4 = > 75% improvement).
Participant‐reported adverse events (short‐term): The mean grade of pain was noted to be 4.49 on the laser‐treated sides and 3.33 on the chemical peeling‐treated sides using a 10‐point scale (0 – 9). Mean erythema lasting days were noted to be 3.30 days on the laser‐treated sides and 12.13 days on the chemical peeling‐treated sides (P value not assessed).
Post‐procedure down time (days): Mean down times were noted to be 3.17 days on the laser‐treated sides (n = 18) and 9.72 days on the chemical peeling‐treated sides (n = 18) (P value not assessed).
7. Fractional laser versus combined chemical peeling plus needling
One parallel‐group study (Leheta 2014b) addressed this comparison in which 39 participants with atrophic acne scarring were randomised into three equal groups, to include the group who received six sessions (four weeks apart) of fractional non‐ablative 1540 nm Er:Glass laser and the comparator who received six sessions (four weeks apart) of chemical peeling with trichloroacetic acid (TCA) 20% combined with skin needling, but we excluded the third arm of this trial. This study did not assess several of our secondary outcomes. See Table 3 for our grading of the evidence.
Primary outcomes
Participant‐reported scar improvement (long‐term): Twelve months after treatment, 9/13 participants in the fractional laser group reported more than 50% improvement in acne scars, and 9/13 participants in the combined chemical peeling plus needling group reported more than 50% improvement in acne scars, using a weighted scale and then a quartile grading scale (0 = minimal improvement < 25%; 1 = mild improvement 25% – 50%; 2 = moderate improvement 51% – 75%; 3 = significant improvement > 75%). No statistically significant difference was reported (RR 1.00, 95% CI 0.60 to 1.67; very low‐quality evidence; Analysis 7.1). We judged this study to be at high risk of detection bias.
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: 1/13 in the combined chemical peeling plus needling group received one session and was then lost to follow‐up; we included this participant in the analyses on an intention‐to‐treat basis. All other participants completed the allocated treatments and were included in the analysis.
7.1. Analysis.
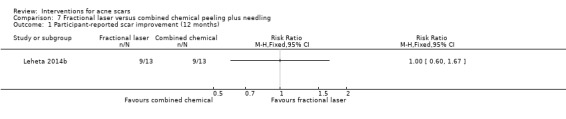
Comparison 7 Fractional laser versus combined chemical peeling plus needling, Outcome 1 Participant‐reported scar improvement (12 months).
Secondary outcomes
Investigator‐assessed scar improvement (long‐term): Twelve months after treatment, acne scarring showed more than 50% improvement in 10/13 participants in the fractional laser group and in 10/13 participants in the combined chemical peeling plus needling group, using a weighted scale and then a quartile grading scale (0 = minimal improvement < 25%; 1 = mild improvement 25% – 50%; 2 = moderate improvement 51% – 75%; 3 = significant improvement > 75%). No statistically significant difference was reported (RR 1.00, 95% CI 0.66 to 1.52; Analysis 7.2).
Participant‐reported adverse events (short‐term): All participants in both groups (n = 25) reported pain, transient oedema and erythema (RR 1.00, 95% CI 0.86 to 1.16; very low‐quality evidence; Analysis 7.3).
7.2. Analysis.
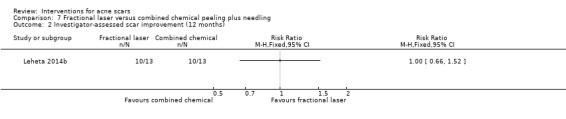
Comparison 7 Fractional laser versus combined chemical peeling plus needling, Outcome 2 Investigator‐assessed scar improvement (12 months).
7.3. Analysis.
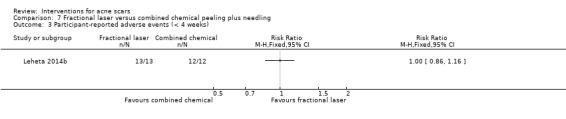
Comparison 7 Fractional laser versus combined chemical peeling plus needling, Outcome 3 Participant‐reported adverse events (< 4 weeks).
8. Chemical peeling versus placebo or no treatment
One parallel group study (Erbağci 2000) addressed this comparison in which 58 women with atrophic acne scarring were randomised into three groups; one group (n = 23) received serial bi‐weekly applied glycolic acid peels with different concentrations in a gradually increasing manner (two‐week intervals), one group (n = 20) received 15% glycolic acid cream daily for 24 weeks, and the remaining group (n = 15) received a placebo cream daily. We combined the first two arms into a single‐arm group who received chemical peeling (n = 43) to be compared with the placebo group. This study did not assess our first primary outcome or several of our secondary outcomes. See Table 4 for our grading of the evidence.
Primary outcomes
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: 48/58 participants completed the study; in the chemical peeling group, nine participants did not complete the study: seven participants withdrew because they were unable to tolerate higher concentrations and longer contact times of the peeling agent, and two were lost to follow‐up. In the placebo group, one participant was lost to follow‐up and none were reported to have serious adverse events. No statistically significant difference was reported (RR 5.45, 95% CI 0.33 to 90.14; very low‐quality evidence; Analysis 8.1). We rated this study at high risk of attrition bias.
8.1. Analysis.
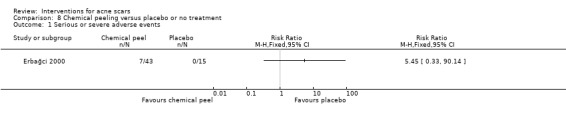
Comparison 8 Chemical peeling versus placebo or no treatment, Outcome 1 Serious or severe adverse events.
Secondary outcomes
Investigator‐assessed scar improvement (short‐term): Final assessment of the results revealed a good response (> 60% grade change from the baseline) in 6/34, a partial response (30% ‐ 60% grade change from the baseline) in 22/34, and minor response (< 30% grade change from the baseline) in 6/34 in the chemical peeling group, while a partial response in 5/14, a minor response in 6/14, and no response in 3/14 were detected in the placebo group, using a 10‐point scale for overall severity of the scars as follows: 0 = no scar; 1 = very mild; 2 ‐ 3 = mild; 4 ‐ 7 = moderate; 8 ‐ 9 = severe; 10 = very severe scar, with a significantly better response in the chemical peeling group (n = 34) (P < 0.05). The reported values of the improvement were the average of the two readings taken (participant and investigator) and could not be used in the analysis.
Participant‐reported adverse events (short‐term): Burning sensation and deep erythema were reported following frosting in 4/34 participants in the chemical peeling group. There were mo reported adverse events in the control group.
Investigator‐assessed adverse events (long‐term): Frosting and whitening were reported in 4/34 participants that were confined to the scar areas. Persistent post‐inflammatory hyperpigmentation lasted for two to three months and prolonged erythema lasting several days were reported in 7/34 participants in the chemical peeling group. There were no reported adverse events in the control group.
9. Chemical peeling versus combined chemical peeling plus any active intervention
One parallel‐group study (Leheta 2014a) addressed this comparison in which 24 participants with atrophic acne scarring were randomised to receive one session of deep peeling using a non‐hydro‐alcoholic solution of oil phenol in 60% concentration formula or the comparator of four sessions (six‐week intervals) of chemical peeling with TCA 20% combined with skin needling. Twenty participants (10 in each group) completed the study and were included in the analyses. This study did not assess several of our secondary outcomes. See Table 5 for our grading of the evidence.
Primary outcomes
Participant‐reported scar improvement (long‐term): Eight months after treatment, all participants (10/10) in the chemical peeling group reported more than a 50% improvement in acne scars, while 80% (8/10) of participants in the chemical peeling plus needling group reported more than a 50% improvement in acne scars, using a weighted scale and then a quartile grading scale (0 = minimal improvement < 25%; 1 = mild improvement 25% – 50%; 2 = moderate improvement 51% – 75%; 3 = significant improvement > 75%). No statistically significant difference was reported (RR 1.24, 95% CI 0.87 to 1.75; very low‐quality evidence; Analysis 9.1). We judged this study to be at high risk of detection bias.
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: 2/12 participants in each group did not receive the allocated treatment after enrolment in the study and were not included in the analyses. Otherwise, all participants (n = 10 in each group) completed the allocated treatments and did not withdraw due to serious adverse effects.
9.1. Analysis.
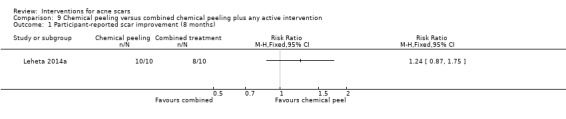
Comparison 9 Chemical peeling versus combined chemical peeling plus any active intervention, Outcome 1 Participant‐reported scar improvement (8 months).
Secondary outcomes
Investigator‐assessed scar improvement (long‐term): Eight months after treatment, acne scarring showed more than 50% improvement in all participants (10/10) in the chemical peeling group and in 80% (8/10) of participants in the chemical peeling plus needling group, using a weighted scale then a quartile grading scale (0 = minimal improvement < 25%; 1 = mild improvement 25% – 50%; 2 = moderate improvement 51% – 75%; 3 = significant improvement > 75%). No statistically significant difference was reported (RR 1.24, 95% CI 0.87 to 1.75; Analysis 9.2).
Participant‐reported adverse events (short‐term): All participants in both groups (n = 20) reported pain either during the session despite the use of topical anaesthetic cream (in the chemical peeling plus needling group) or after recovery from the general anaesthesia (in the chemical peeling group). All participants in both groups (n = 20) reported transient erythema which lasted for more than one month in the chemical peeling group and for only two to three days in the chemical peeling plus needling group. No statistically significant difference was reported (RR 1.00, 95% CI 0.83 to 1.20; very low‐quality evidence; Analysis 9.3).
Investigator‐assessed adverse events (long‐term): All participants (10/10) in the chemical peeling group showed erythema for three to four months and pigmentation for six months. Two of 10 participants (20%) in this group had persistent erythema for six months. None of the participants in the chemical peeling plus needling group showed any adverse events one month after the procedure. There was a statistically significant difference (P < 0.001, Fisher's exact test) in favour of chemical peeling plus needling (RR 21.00, 95% CI 1.40 to 315.98; Analysis 9.4).
9.2. Analysis.
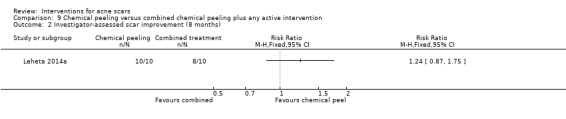
Comparison 9 Chemical peeling versus combined chemical peeling plus any active intervention, Outcome 2 Investigator‐assessed scar improvement (8 months).
9.3. Analysis.
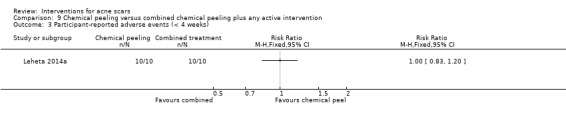
Comparison 9 Chemical peeling versus combined chemical peeling plus any active intervention, Outcome 3 Participant‐reported adverse events (< 4 weeks).
9.4. Analysis.
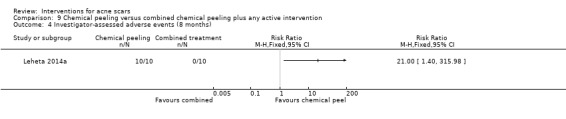
Comparison 9 Chemical peeling versus combined chemical peeling plus any active intervention, Outcome 4 Investigator‐assessed adverse events (8 months).
10. Chemical peeling versus needling
One parallel‐group study (Leheta 2011) addressed this comparison in which 30 participants with atrophic acne scarring were randomised to receive four sessions (four‐week intervals) of chemical peeling using full‐strength trichloroacetic acid (100% TCA) CROSS or the comparator of four sessions (four‐week intervals) of skin needling using a dermaroller. Three out of 15 participants in the peeling group received one treatment session and were then lost to follow‐up so were not included in the analyses. This study did not assess several of our secondary outcomes. See Table 6 for our grading of the evidence.
Primary outcomes
Participant‐reported scar improvement (short‐term): One month after the last treatment session, 9/12 participants in the chemical peeling group reported more than a 50% improvement in acne scars, while 10/15 of participants in the needling group reported more than a 50% improvement in acne scars, using a weighted scale and then a quartile grading scale (0 = minimal improvement < 25%; 1 = mild improvement 25% – 50%; 2 = moderate improvement 51% – 75%; 3 = significant improvement > 75%). No statistically significant difference was reported between the interventions (RR 1.13, 95% CI 0.69 to 1.83; very low‐quality evidence; Analysis 10.1). We rated this study at high risk of detection bias.
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: Apart from the three out of 15 participants in the peeling group who received one treatment session and were then lost to follow‐up, all other participants (n = 27) completed the allocated treatments and did not withdraw due to serious adverse events. We rated this study at high risk of attrition bias.
10.1. Analysis.
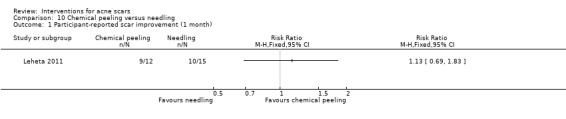
Comparison 10 Chemical peeling versus needling, Outcome 1 Participant‐reported scar improvement (1 month).
Secondary outcomes
Investigator‐assessed scar improvement (short‐term): One month after the last treatment session, 11/12 participants in the chemical peeling group reported more than a 50% improvement in acne scars, while 12/15 participants in the needling group reported more than a 50% improvement in acne scars, using a weighted scale and then a quartile grading scale (0 = minimal improvement < 25%; 1 = mild improvement 25% – 50%; 2 = moderate improvement 51% – 75%; 3 = significant improvement > 75%). No statistically significant difference was reported between the interventions (RR 1.15, 95% CI 0.84 to 1.55; Analysis 10.2).
Participant satisfaction: One month after the last treatment, 9/12 participants in the chemical peeling group and 10/15 participants in the needling group were satisfied with the treatment. No statistically significant difference (P = 0.696, Fisher's exact test) was reported between the interventions (RR 1.13, 95% CI 0.69 to 1.83; very low‐quality evidence; Analysis 10.3).
Participant‐reported adverse events (short‐term): All participants in both groups (n = 27) experienced pain which showed a higher mean of 5.4 (SD 1.9) in the needling group than the mean of 3.8 (SD 1.6) detected in the peeling group. All participants in both groups (n = 27) also developed transient erythema which lasted for a mean of 15.9 (SD 4.3) days in the chemical peeling group and for a mean of 3 (SD 0.8) days in the needling group. No statistically significant difference was reported between the interventions (RR 1.00, 95% CI 0.87 to 1.15; very low‐quality evidence; Analysis 10.4).
Investigator‐assessed adverse events (short‐term): Transient post‐inflammatory hyperpigmentation lasting for one month occurred in 50% of participants (6/12) in the peeling group. None of the participants in the needling group showed any side effect one month after the treatment session (P = 0.003, Fisher's exact test) in favour of needling (RR 16.00, 95% CI 0.99 to 258.36; low‐quality evidence; Analysis 10.5).
Post‐procedure down time (days): The overall mean post‐procedure down time was 9.6 (SD 3.1) days in the peeling group (n = 12) and was 3.7 (SD 1) days in the needling group (n = 15). There was a statistically significant difference in favour of needling (MD 5.90, 95% CI 4.07 to 7.73; Analysis 10.6).
10.2. Analysis.
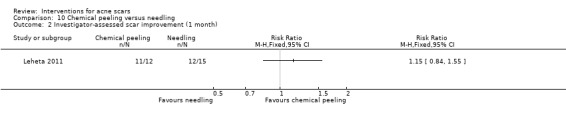
Comparison 10 Chemical peeling versus needling, Outcome 2 Investigator‐assessed scar improvement (1 month).
10.3. Analysis.
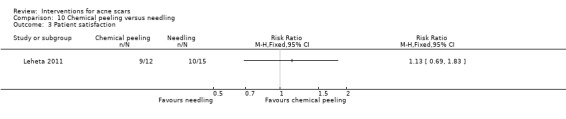
Comparison 10 Chemical peeling versus needling, Outcome 3 Patient satisfaction.
10.4. Analysis.
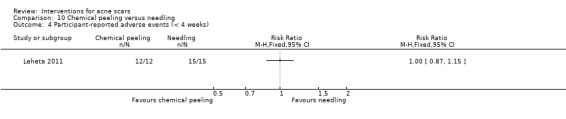
Comparison 10 Chemical peeling versus needling, Outcome 4 Participant‐reported adverse events (< 4 weeks).
10.5. Analysis.
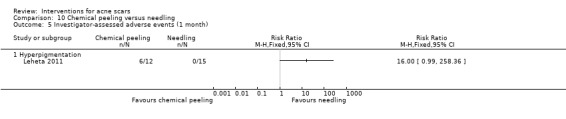
Comparison 10 Chemical peeling versus needling, Outcome 5 Investigator‐assessed adverse events (1 month).
10.6. Analysis.
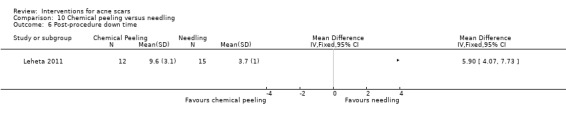
Comparison 10 Chemical peeling versus needling, Outcome 6 Post‐procedure down time.
11. Needling versus placebo or no treatment
One within‐individual study (Alam 2014) with 20 participants (of which 15 were analysed) compared needling which was randomly performed on one side (study area) for three sessions (two‐week intervals) while on the other side topical anaesthetic cream only was massaged onto the control area through three treatment visits. This study did not assess several of our secondary outcomes. See Analysis 11.1 for a precis of our findings.
11.1. Analysis.
Comparison 11 Needling versus placebo or no treatment, Outcome 1 Within‐individual studies.
| Within‐individual studies | |||
|---|---|---|---|
| Study | Interventions | Summary Outcomes | Comment |
| Alam 2014 | One side: Needling Other side: untreated control | Improvement of acne scars (6 months): Participants perceived a 41% mean improvement in overall scar appearance on the treated side. In the needling group, scar scores were significantly lower at 6 months compared with baseline (MD 3.4, 95% CI 0.2 to 6.5; P = 0.03). In the untreated control group, mean scar scores did not vary significantly from baseline at 6 months (MD 0.4, 95% CI −2.8 to 3.5; P > 0.99). Participant satisfaction: Most participants were very satisfied with their procedure. Any adverse events: No adverse events were reported. The mean pain rating was 1.08. |
Alam 2014 did not assess the primary outcome ‘Participant‐reported failure of treatment’ nor the secondary outcomes ‘Investigator‐reported failure of treatment’, ‘Quality of life’ and ‘Post‐procedure down time’. |
Primary outcomes
Participant‐reported scar improvement (short‐term): Participants (n = 15) reported a 41% mean improvement in acne scars on the treated side.
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: No adverse events were reported during the whole study.
Secondary outcomes
Investigator‐assessed scar improvement (short‐term): In the needling group (n = 15), scar scores were significantly lower at six months compared with baseline (mean difference (MD) 3.4, 95% CI 0.2 to 6.5; P = 0.03). In the untreated control group (n = 15), mean scar scores did not vary significantly from baseline at six months (MD 0.4, 95% CI −2.80 to 3.50; P > 0.99) using the Goodman and Baron global scarring grading system (Goodman 2006a).
Participant satisfaction: "Most participants" (no number reported) were very satisfied with their procedure.
Participant‐reported adverse events (short‐term): The mean pain rating was 1.08 of 10 using a VAS. Mild transient erythema and oedema, which were not classified as adverse events and hence not formally tracked, were routinely reported by participants after treatments.
Investigator‐assessed adverse events (short‐term): Mild transient erythema and oedema were seen in all participants (n = 15); these were not classified as adverse events and hence not formally tracked, but were routinely observed by the investigator after treatments.
12. Injectable fillers versus placebo or no treatment
One parallel‐group study (Karnik 2014) and one within‐individual study (Munavalli 2013) made these comparisons. See Table 7 for our grading of the evidence.
Karnik 2014 randomised 157 participants with atrophic acne scars. Participants received injections with polymethylmethacrylate (PMMA) suspended in bovine collagen, or the comparator which was saline injections administered in a similar manner. One hundred and forty‐seven participants (97 in the injectable filler group and 50 in the placebo group) received at least one injection and were included in the analyses. This study did not assess several of our secondary outcomes.
Primary outcomes
Participant‐reported scar improvement (short‐term): The Subject Global Aesthetic Improvement Scale score demonstrated a greater response with 77% (75/97) of participants in the injectable filler group rated as improved compared with 42% (21/50) in the placebo group (P < 0.05). There was a statistically significant difference in favour of injectable filler (RR 1.84, 95% CI 1.31 to 2.59; moderate‐quality evidence; Analysis 12.1). We rated this study at low risk of detection bias.
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: No serious adverse events were reported during the whole study.
12.1. Analysis.
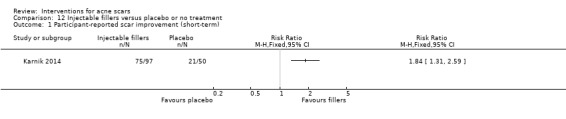
Comparison 12 Injectable fillers versus placebo or no treatment, Outcome 1 Participant‐reported scar improvement (short‐term).
Secondary outcomes
Investigator‐assessed scar improvement (short‐term): The Physician Global Aesthetic Improvement Scale score demonstrated a greater response with 84% (82/97) in favour of injectable filler compared with 54% (27/50) in the placebo group (RR 1.57, 95% CI 1.20 to 2.05; Analysis 12.2).
Participant satisfaction: Participants showed an elevated level of satisfaction through their scores on the Subject Assessment of Scar Correction. A total of 84% (82/97) of participants in the injectable filler group were satisfied or better, compared with 52% (26/50) in the placebo group (Karnik 2014). There was a statistically significant difference in favour of injectable filler (RR 1.63, 95% CI 1.23 to 2.15; moderate‐quality evidence; Analysis 12.3).
Participant‐reported adverse events (short‐term): A list of reactions were noted with the injectable filler injections in 2.1% (2/97) of participants compared with 2% (1/50) in the placebo group, including erythema, swelling, bruising, pain, itching, lumps or bumps, and discolouration. Almost all reports were mild or moderate in severity with an average duration of from two days for pain and itching up to a maximum of six days for discolouration. No significant difference in the adverse events was noted between the groups (RR 1.03, 95% CI 0.10 to 11.10; low‐quality evidence; Analysis 12.4).
Investigator‐assessed adverse events (short‐term): Adverse events were reported in 17/97 participants in the injectable filler group and in 13/50 participants in the control group. The most commonly reported adverse events included injection‐site pain, injection‐site tenderness, and swelling. No significant difference in adverse events was noted between the groups (RR 0.67, 95% CI 0.36 to 1.27; low‐quality evidence; Analysis 12.5).
Investigator‐assessed adverse events (long‐term): Hyperpigmentation, hypopigmentation, hypertrophic scarring, and granuloma formation were not reported during the study. No significant difference in adverse events was noted between the groups.
12.2. Analysis.
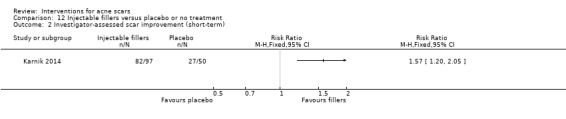
Comparison 12 Injectable fillers versus placebo or no treatment, Outcome 2 Investigator‐assessed scar improvement (short‐term).
12.3. Analysis.
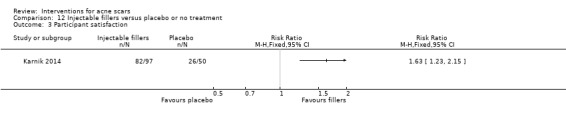
Comparison 12 Injectable fillers versus placebo or no treatment, Outcome 3 Participant satisfaction.
12.4. Analysis.
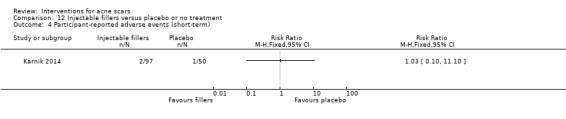
Comparison 12 Injectable fillers versus placebo or no treatment, Outcome 4 Participant‐reported adverse events (short‐term).
12.5. Analysis.
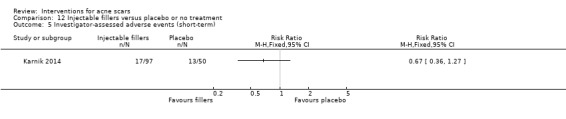
Comparison 12 Injectable fillers versus placebo or no treatment, Outcome 5 Investigator‐assessed adverse events (short‐term).
Munavalli 2013 was a within‐individual trial in which one cheek was randomised to receive autologous fibroblasts injected into the dermis at a maximum dose of 2 ml per treatment for three treatments (14 days apart), while the other cheek received vehicle control (dye‐free, protein‐free cell culture medium) injected into the dermis at a maximum dose of 2 ml per treatment for three treatments (14 days apart). Seven out of 109 treated participants did not continue the treatment plan: one person declared reasons unrelated to adverse events, and six were lost to follow‐up. No data were available for three participants, so 99 participants completed and were analysed. This study did not assess several of our secondary outcomes. See Analysis 12.6 for a precis of our findings.
12.6. Analysis.
Comparison 12 Injectable fillers versus placebo or no treatment, Outcome 6 Within‐individual studies.
| Within‐individual studies | |||
|---|---|---|---|
| Study | Interventions | Summary Outcomes | Comment |
| Munavalli 2013 | One side: Injectable filler using autologous fibroblasts injected into the dermis Other side: vehicle control (dye‐free, protein‐free cell culture medium) injected into the dermis |
Improvement of acne scars: More than twice as many participants rated the injectable filler treated area with a 2‐point or greater improvement than the area receiving vehicle control (43% vs 18%). Evaluators rated 59% of the injectable filler–treated sides with a 1‐point or greater improvement on the evaluator scale compared with 42% of the sides receiving vehicle control. Side effects: No participants experienced serious adverse events, discontinued treatment, or withdrew from the study as a result of a treatment‐emergent adverse event. The most common adverse events were treatment area erythema (occurring in 11.1% of participants) and swelling (occurring in 10.1% of participants). |
Munavalli 2013 did not report the secondary outcomes ‘Participant satisfaction’, ‘Quality of life’, ‘Participant‐reported adverse events’ and ‘Post‐procedure down time’. |
Primary outcomes
Participant‐reported scar improvement (short‐term): Participants reported that 43% (n = 43) of the injectable filler‐treated sides showed a two‐point or greater improvement compared with 18% (n = 18) of the vehicle control‐treated sides using a five‐point scale for the acne scar assessment (‐2 = very dissatisfied; ‐1 = dissatisfied; 0 = somewhat satisfied; +1 = satisfied; +2 = very satisfied), co‐primary endpoint P < 0.001. We judged this study to be at low risk of detection bias.
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: There were no reported adverse effects severe enough to cause participants to withdraw from the study.
Secondary outcomes
Investigator‐assessed scar improvement (short‐term): 59% of the injectable filler–treated sides (n = 58) showed one‐point or greater improvement on the used scale compared with 42% of the vehicle control‐treated sides (n = 42), using a five‐point evaluator live acne scar assessment scale (0 = clear; 1 = very mild; 2 = mild; 3 = moderate; 4 = severe), co‐primary endpoint P = 0.01. Based on the three independent photographic reviewers' (IPRs) scores, each of the three reviewers repeatedly classified the cheeks injected with dermal filler as statistically significantly more improved than the cheeks treated with the placebo control, using a five‐point scale (–2 = much worse; –1 = worse; 0 = no change; +1 = improved; +2 = much improved).
Investigator‐assessed adverse events (short‐term): The reported adverse events showed comparable incidence between both interventions. The most reported adverse effects were erythema in 11.1% of participants (n = 11) and swelling in 10.1% (n = 10). All treatment area‐related adverse effects showed mild or moderate severity.
Investigator‐assessed adverse events (long‐term): No clinically meaningful changes were observed in skin pigmentation or evidence of hypertrophic scarring in either treated area.
13. Injectable fillers versus subcision
One within‐individual study (Sage 2011) with 10 participants made this comparison, in which one half of the face was injected with the injectable filler using a natural‐source porcine collagen (NSPC) filler for a single session, while the other half was treated with subcision using an 18‐gauge Nokor subcision needle for a single session. This study did not assess several of our secondary outcomes. See Analysis 13.1 for a precis of our findings.
13.1. Analysis.
Comparison 13 Injectable fillers versus subcision, Outcome 1 Within‐individual studies.
| Within‐individual studies | |||
|---|---|---|---|
| Study | Interventions | Summary Outcomes | Comment |
| Sage 2011 | One side: Injectable filler using a natural source porcine collagen (NSPC) filler Other side: Subcision |
Participant‐reported scar improvement (6 months): Participants rated subcision (3.9) higher than NSPC injectable filler (3.5) for global improvement (P = 0.12). Physician assessment of the overall aesthetic improvement revealed a higher mean score for global improvement with NSPC injectable filler (3.05) than with subcision (2.95) (P = 0.69). Participant‐reported adverse events (1 week): The most significant adverse effect reported was bruising in participants treated with subcision. Subcision had a higher incidence and mean severity of bruising (2.2) than NSPC injection (0.7) (P = 0.007) Participant‐reported adverse events (6 months): Participants rated lumpiness from subcision (mean 3.4) as better than NSPC injectable filler (mean 2.9) (P = 0.15). Investigator‐assessed adverse events (1 week): A higher mean severity of bruising with subcision (1.7) than with NSPC injection (1.1) (P = 0.09). |
Sage 2011 did not assess the secondary outcomes ‘Participant satisfaction’, ‘Quality of life’ or ‘Post‐procedure down time'. |
Primary outcomes
Participant‐reported scar improvement (short‐term): Six months after treatment, participants (n = 10) reported a higher global improvement rate with subcision (3.9) than NSPC injectable filler (3.5), with no significant difference between either intervention (P = 0.12), using a scale for the overall aesthetic improvement from 1 to 5 "(1 = worse than before treatment; 2 = no change; 3 = minimal disappearance; 4 = moderate disappearance; 5 = complete disappearance)" (Sage 2011). We rated this study at high risk of detection bias.
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: All 10 participants completed the six‐month follow‐up visit.
Secondary outcomes
Investigator‐assessed scar improvement (short‐term): Physician assessment of the overall aesthetic improvement revealed that NSPC injectable filler has a higher global improvement mean score (3.05; n = 10) than subcision (2.95; n = 10), with no significant difference between the interventions (P = 0.69) using a scale from 1 to 5 (1 = worse than before treatment; 2 = no change; 3 = minimal disappearance; 4 = moderate disappearance; 5 = complete disappearance).
Participant‐reported adverse events (short‐term): Participants graded adverse events of pain, erythema, swelling, discolouration, bruising, and lumpiness on a tolerability scale from 0 to 3. Participants rated bruising from subcision (mean severity 2.2) as significantly worse than from NSPC injection (mean severity 0.7; P = 0.007). Participants reported that lumpiness from subcision (mean 3.4) was significantly better than from NSPC injectable filler (mean 2.9; P = 0.15) using a scale from 1 to 5 (1 = worse than before treatment; 2 = no change; 3 = minimal disappearance; 4 = moderate disappearance; 5 = complete disappearance). Discolouration was equally reported for both treatments (mean 3.4, P > 0.99).
Investigator‐assessed adverse events (short‐term): A higher mean severity of bruising was reported with subcision (1.7) than with NSPC injection (1.1) with no significant difference between the interventions (P = 0.09) using a scale from 0 to 3 (0 = no symptoms; 1 = mild symptoms; 2 = moderate symptoms; 3 = severe symptoms). Severe adverse events were not stated for either treatment.
14. Combined microdermabrasion plus ALA‐PDT versus combined microdermabrasion plus placebo‐PDT
One within‐individual study (Linkner 2014) with five participants made this comparison in which one cheek was randomised to receive a solution of 20% δ‐aminolevulinic acid (ALA) while the other cheek received a vehicle solution alone applied topically to the face. All the lesions were illuminated with 417 nm blue light (Blu‐U Blue Light Photodynamic Therapy Illuminator) after full‐face treatment with microdermabrasion for five sessions (four‐week interval). Six participants were enrolled, of whom five completed the study. This study did not assess any of our primary outcomes or several of our secondary outcomes. See Analysis 14.1 for a precis of our findings.
14.1. Analysis.
Comparison 14 Combined microdermabrasion plus ALA‐PDT versus combined microdermabrasion plus placebo‐PDT, Outcome 1 Within‐individual studies.
| Within‐individual studies | |||
|---|---|---|---|
| Study | Interventions | Summary Outcomes | Comment |
| Linkner 2014 | One side: microdermabrasion plus 20% δ‐aminolevulinic acid with PDT (ALA‐PDT) Other side: microdermabrasion plus vehicle solution with PDT (vehicle‐PDT) |
Improvement of acne scars (5 months) 80% of the participants displayed more improvement in scarring on the ALA split face versus the vehicle split face. Participant satisfaction (5 months): 80% of participants appreciated an improvement in the acne scarring. Side effects (5 months): No side effects were noted. |
Linkner 2014 did not assess the primary outcomes ‘Participant‐reported scar improvement’, ‘Serious adverse effects’, nor the secondary outcomes ‘Quality of life’ and ‘Post‐procedure down time’. |
Secondary outcomes
Investigator‐assessed scar improvement (short‐term): At the end of the study, 80% of the participants (4/5) showed more improvement in scarring on the combined microdermabrasion plus ALA‐PDT split‐face versus the combined microdermabrasion plus vehicle‐PDT split‐face using Physician’s Global Assessment of Acne Scarring scale (P value not assessed).
Participant satisfaction: At the end of the study, 80% of participants (4/5) appreciated an improvement in the acne scarring.
Participant‐reported adverse events (short‐term): Linkner 2014 mentioned that the study would assess adverse effects, including pain, phototoxic parameters and pigmentary changes using a 10‐point scale (0 = none; 1 – 3 = mild; 4 – 6 = moderate; 7 – 9 = severe) during and immediately after each treatment, but no side effects were reported.
Investigator‐assessed adverse events (short‐term): Linkner 2014 mentioned that the study would assess adverse effects, including pain, phototoxic parameters and pigmentary changes using a 10‐point scale (0 = none; 1 – 3 = mild; 4 – 6 = moderate; 7 – 9 = severe) during and immediately after each treatment, but no side effects were reported.
Five split‐face studies
In this section there are five more split‐face studies (Cho 2010; Lee 2009; Manuskiatti 2013; Min 2009; Tanzi 2004) comparing two interventions for acne scarring but which could not be incorporated in our comparisons, so are described narratively.
Manuskiatti 2013 compared fractional ablative Er:YAG laser to fractional ablative CO₂ laser. Both comparators are in the same category as fractional ablative laser. Manuskiatti 2013 was a within‐individual trial (24 enrolled and 20 completed the study), in which one facial half received fractional Er:YAG laser treatment and the other facial half received fractional CO₂ laser treatment, each group receiving two sessions at two‐month intervals. Participants were followed up for six months after the final session. This study did not assess several of our secondary outcomes.
Primary outcomes
Participant‐reported scar improvement (short‐term; six months): Participants graded their global improvement of acne scars on a quartile scale (slightly better = < 25%; fair = 25% ‐ 50%; good = 51% ‐ 75%; excellent = > 76%). Accordingly, 70% (14/20) and 60% (12/20) of Er:YAG and CO₂ laser sites respectively were rated by participants as showing more than a 50% improvement, with no statistically significant difference between interventions (P = 0.47). We judged this study to be at unclear risk of detection bias.
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: None of the three of 24 enrolled participants who were withdrawn from the study due to scheduling conflicts was affected by serious adverse events. However, it remains questionable why the fourth participant was withdrawn, as he could not be contacted during follow‐up.
Secondary outcomes
Investigator‐assessed scar improvement (short‐term; six months): A quartile grading scale (0 = < 25%; 1 = 25% ‐ 50%; 2 = 51% ‐ 75%; 3 = > 75% improvement) was used by a blinded dermatologist to evaluate the global acne scars improvement. Six months after treatment, 55% (11/20) and 65% (13/20) of Er:YAG and CO₂ laser sites respectively were rated as having more than a 50% improvement, with no statistically significant difference between the interventions (P = 0.87).
Participant‐reported adverse events (short‐term: less than four weeks): Pain was largely well tolerated by all participants, but they reported a significantly higher pain score on the CO₂ laser than on the Er:YAG laser site (P = 0.001). Average pain scores described by the participants were 3.2 (SD 1.4) and 5.8 (SD 2.0) on the site treated with Er:YAG and CO₂ laser respectively, using a 10‐point pain scale (0 = no pain to 10 = severe pain). Pain lasted for an average of three hours.
Investigator‐assessed adverse events: Adverse effects included moderate to marked erythema, mild to moderate oedema on both treated sites, followed by superficial crusting. Post‐inflammatory hyperpigmentation occurred in 7/20 and 10/20 of participants at the Er:YAG and CO₂ laser sites respectively (P = 0.52).
Post‐procedure down time: Erythema and oedema persisted for 24 hours on both treated sites. Superficial crusting subsequently occurred and completely sloughed off in an average of 3.6 and 3.3 days with no statistically significant difference on both Er:YAG and CO₂ laser‐treated sides respectively (P = 0.80).
Cho 2010 was a within‐individual trial of eight participants in which one side of the face was randomised to receive one session of non‐ablative 1550‐nm erbium‐doped fractional photothermolysis system (FPS) and the other side of the face was treated with 10,600‐nm CO₂ fractional laser system (CO₂ FS). This study did not assess our first primary outcome or several of our secondary outcomes.
Primary outcomes
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: The eight treated participants completed the study follow‐up period.
Secondary outcomes
Investigator‐assessed scar improvement (short‐term; three months): A quartile grading scale was used by blinded investigators to assess global improvement of acne scars as follows: grade 1, < 25% = minimal to no improvement; grade 2, 26% to 50% = moderate improvement; grade 3, 51% to 75% = marked improvement; grade 4, > 75% = near‐total improvement. Three months after treatment, the mean grade of improvement was 2.0 ± 0.5 for FPS and 2.5 ± 0.8 for CO₂ FS. One of eight participant showed more than a 50% improvement after a single session of FPS, versus three of eight participant after a single session of CO₂ FS with no statistically significant difference (P = 0.158).
Participant satisfaction: Participants evaluated their overall levels of satisfaction with treatment results using the following scale: very satisfied, satisfied, slightly satisfied and unsatisfied) with separate evaluations of each side of the face. The overall satisfaction levels were not significantly different (P = 0.105). Two of eight participants were satisfied and none of eight very satisfied after FPS treatment, versus four of eight satisfied and two of eight very satisfied after CO₂ FS treatment.
Participant‐reported adverse events (short‐term: less than four weeks): Side effects included pain during the laser treatment, crusting or scaling after treatment, redness after therapy, fluid retention, hyperpigmentation after therapy, bleeding and oozing from the treated sites, and worsening of inflammatory acne lesions (Cho 2010). Relative pain scores were evaluated using 10‐cm VAS, with 0 being ‘no pain’ and 10 being ‘extremely painful’. The mean VAS pain score was significantly lower (3.9 ± 2.0) with the FPS than with the CO₂ FS treatment (7.0 ± 2.0; P = 0.012).
Post‐procedure down time: On the area of the face treated by fractional non‐ablative laser, the mean period of crusting and scaling after treatment was 2.3 ± 2.9 days and that of erythema after treatment was 7.5 ± 5.7 days. On the area of the face treated by CO₂ FS, the mean duration of crusting and scaling after treatment was 7.4 ± 2.4 days and that of erythema after treatment was 11.5 ± 5.2 days. This variation in the time period of crusting or scaling after treatment was statistically significant (P = 0.006), but the variation in the length of erythema after treatment (P = 0.145) was not (Cho 2010).
Lee 2009 was a within‐individual trial of 18 participants in which one facial half received non‐fractional non‐ablative pulsed dye laser (PDL) treatment and the other facial half received 1064‐nm long pulsed neodymium;yttrium‐aluminium‐garnet (NdYAG) laser treatment, four sessions at two‐week intervals. Participants were followed up for eight weeks after the final session (a total 14 weeks). This study did not assess our first primary outcome or several of our secondary outcomes.
Primary outcomes
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: All participants completed the allocated treatments and were included in the analyses.
Secondary outcomes
Investigator‐assessed scar improvement (short‐term; eight weeks): The progress of acne scars was measured by evaluating the degrees of improvement according to the sort of scar and the ECCA scores (Lee 2009). ECCA scores significantly reduced following PDL (18.3% improvement) and Nd:YAG (18.7% improvement) treatments (P = 0.005 and P = 0.011 respectively), but these improvements were not statistically significantly different between both interventions (P value not mentioned).
Participant satisfaction: Participant subjective satisfaction scores were established at every appointment by use of a 0 (neutral) to 10 (highly satisfied) scale (Lee 2009). Satisfaction scores increased throughout the therapy sessions with no statistically significant difference between the two groups (P value not mentioned).
Participant‐reported adverse events (less than four weeks): Reported adverse events included transient pain, erythema, and oedema in the treated areas.
Tanzi 2004 was a within‐individual trial of 20 participants in which one facial half received non‐fractional non‐ablative 1320‐nm long pulsed NdYAG laser treatment and the other facial half received non‐fractional non‐ablative 1450‐nm diode laser treatment, each arm receiving three sessions at four‐week intervals. Participants were followed up for 12 months after the final session. This study did not assess any of our primary outcomes or several of our secondary outcomes.
Secondary outcomes
Investigator‐assessed scar improvement (long‐term; 12 months): The degree of improvement in the quality of skin texture was evaluated on clinical photographs using a quartile grading scale (1: less than 25% = minimal to no improvement; 2: 25% ‐ 50% = moderate improvement; 3: 51% ‐ 75% = marked improvement; 4: > 75% = near total improvement). Higher average clinical scores were seen on the 1450‐nm diode laser‐treated facial halves at each visit in comparison to NdYAG laser‐treated half (at six months: 1.81 versus 1.67, and at 12 months: 1.34 versus 1.13 respectively) (P value not reported).
Participant satisfaction: The participants recorded how satisfied they were on a scale of one (lowest) to 10 (highest) for each treated half (Tanzi 2004). The mean satisfaction score was higher in the 1450‐nm diode than the Nd:YAG arm (5.7 versus 4.6 respectively) (P value not reported).
Participant‐reported adverse events (short‐term; less than four weeks): Discomfort during treatment was reported by participants who were less satisfied with the 1450‐nm diode laser treatment.
Investigator‐assessed adverse events: Post‐treatment erythema was seen in all participants studied, and post‐inflammatory hyperpigmentation was observed in 4/20 versus 2/20 participants treated with 1450‐nm diode and 1320‐nm Nd:YAG lasers respectively (P value not reported).
Post‐procedure down time: Erythema lasted for 24 hours versus six hours after treatment with 1450‐nm diode and 1320‐nm Nd:YAG lasers respectively (P value not reported).
Min 2009 was a within‐individual trial of 19 participants in which one facial half received long pulsed Nd:YAG laser treatment and the other facial half received a combined 585/1064‐nm laser treatment, each half receiving four sessions at two‐week intervals. Participants were followed up for eight weeks after the final session (total 14 weeks). This study did not assess our first primary outcome or several of our secondary outcomes.
Primary outcomes
Participants with adverse effects serious or severe enough to have caused their withdrawal from the study: All participants completed the allocated treatments and were included in the analyses.
Secondary outcomes
Investigator‐assessed scar improvement (short‐term; eight weeks): Acne scar improvements were quantified by assessing the degrees of improvement according to scar types and the ECCA scores. ECCA scores significantly reduced following the Nd:YAG (27% improvement) and the combined 585/1064‐nm (32.3% improvement) treatments (P = 0.001 for both). These improvements were not statistically significantly different between the interventions (P value not reported).
Participant satisfaction: Participant subjective satisfaction scores were determined at each visit by use of a 0 (neutral) to 10 (highly satisfied) scale. For Nd:YAGlasertreatment, participant satisfaction scores increased from 0 (baseline) to 4.9 at eight weeks after final treatment. For combined 585/1064‐nm laser treatment, participant satisfaction scores increased from 0 (baseline) to 5.3 at eight weeks after final treatment. There was no statistically significant difference between the two treatment methods (P value not reported).
Participant‐reported adverse events (less than four weeks): Adverse events were only transient pain, erythema, and oedema in both treated areas.
Discussion
Summary of main results
Our searches identified 216 references for potential inclusion, of which 24 trials met our inclusion criteria, with a total of 789 participants. We included trials from each of our eight intervention groups, and in all cases the effects of an intervention were assessed by a single RCT, so meta‐analyses were not possible.
Only 14 trials included our primary efficacy outcome measure 'Participant‐reported scar improvement'. Twenty trials included our primary outcome 'Participants with adverse effects serious or severe enough to have caused their withdrawal from the study'. Eight trials included our secondary outcome 'Participant satisfaction'; however, none of our included studies looked at quality of life. All the studies except one included some information about our secondary outcomes 'Participant‐reported short‐term adverse events' and 'Investigator‐assessed short‐term adverse events'. Of the 24 included trials, 21 included only atrophic acne scars, two studies (Bernstein 2001; Lee 2011) did not specify the types of acne scars, and only one study (Alam 2014) included both atrophic and hypertrophic acne scars. We did not find any trials that included information about acne scars on the back.
Evidence from one study of 64 people showed that fractional laser given for four monthly sessions improves acne scars more than non‐fractional non‐ablative laser at week 24. For the secondary outcome, 'Investigator‐assessed adverse effects' post‐inflammatory hyperpigmentation lasting for two to three weeks was reported by 6/32 and 10/32 participants treated with fractional laser and non‐fractional non‐ablative laser respectively.
One study in 40 people showed that both fractional laser and fractional radiofrequency given for three monthly sessions improves acne scars by week eight.
A study in 26 people showed that both fractional laser and chemical peeling combined with skin needling given for six monthly sessions improves acne scars by week 48. For the secondary outcomes, all participants in both groups reported pain, transient oedema and erythema for less than four weeks.
A study in 58 people comparing chemical peeling to placebo showed that chemical peeling given for 24 weeks had no severe adverse events that caused participants to withdraw from the study; however, seven participants withdrew because they were unable to tolerate the peeling agent.
One study in 20 people showed that both chemical peeling given for one session and chemical peeling combined with skin needling given for four sessions improve acne scars by week 32. For the secondary outcomes, all participants in both groups reported transient erythema which lasted for four weeks in the chemical peeling group and only for two to three days in the chemical peeling plus needling group, with no significant difference related to short‐term adverse events.
One study in 27 people showed that both chemical peeling and skin needling given for four monthly sessions improves acne scars by week four. For the secondary outcomes, short‐term adverse events showed no significant difference between the interventions. For our outcome ‘Participant satisfaction’ participants were satisfied with both chemical peeling and skin needling.
A study in 147 people showed that injectable filler given for one session improves atrophic acne scars by week 24. The Global Aesthetic Improvement Scale reported a significant difference in favour of injectable filler with 77% of participants responding as being improved compared to 42% for the placebo group. For the secondary outcomes, no significant difference in the adverse events was noted between the groups. For our outcome ‘Participant satisfaction’ participants were more satisfied with injectable filler.
We do not have sufficient evidence to determine the effects or the safety of other included interventions such as subcision, combined fractional laser with PRP, combined fractional laser with punch elevation, and combined microdermabrasion plus ALA‐PDT in acne scars.
Overall completeness and applicability of evidence
All the included studies found for this review lack some information about trial methodology and detailed data for some of the reported outcomes. Several did not compare the outcome data between the two treatment groups, so that the clinical significance of the results was unclear.
Quality of the evidence
We judged many studies included in this review as having an unclear or high risk of bias for allocation concealment and blinding of participants. We therefore recommend caution in the interpretation of the results and in the extrapolation of the effects of the interventions. Most of the included studies were underpowered for their primary outcomes as well as for uncommon adverse events.
Although acne scarring is a common condition, the total number of all participants enrolled was only 789 people in 24 trials. The evidence for our main outcomes ‘Participant‐reported scar improvement', 'Participant‐reported adverse events' and 'Participantnt satisfaction’ is drawn from studies at high risk of bias due to lack of blinding of participants. There were 14 comparisons of seven interventions and four combinations of interventions, whether compared to each other, to placebo or to no treatment. This clearly means that each intervention was not compared in enough trials and among enough individuals. Collectively, these factors point to a low quality of evidence. We downgraded the quality of evidence to 'very low' for the main outcomes, due mainly to unknown consistency or imprecision because the OIS was not met, low occurrence of events, or 95% CIs around the estimate of effect which included both no effect and appreciable benefit or harm.
There were not enough studies to conduct the planned subgroup analyses. In future updates, we plan to conduct subgroup analyses classifying whole trials by interaction tests and to carry out sensitivity analysis to explore the effects of fixed‐effect or random‐effects analyses for outcomes with statistical heterogeneity between studies.
Potential biases in the review process
We have taken care to try and eliminate bias; however, it is always possible, although unlikely, that one or more trials have been missed, in journals not covered by the databases that we searched, or in a journal's correspondence section. With so few data in the review at present, any missing trials may have a greater potential to change the review conclusions. The fact that nine studies have not yet been incorporated may be a source of potential bias.
Agreements and disagreements with other studies or reviews
There have been no systematic reviews of acne scarring treatment apart from Jordan 2000, who conducted a systematic review to assess the effects of laser resurfacing for treating facial acne scars, but which found no RCTs where laser intervention was compared to either placebo or to a different type of laser. Most of the studies found were poor‐quality case series with small numbers of participants. Jordan 2000 could not assess the effectiveness of lasers for treating atrophic acne scars. This conclusion is in agreement with our review, which failed to find sufficient evidence from RCTs to support laser therapy for acne scars.
Sánchez Viera 2015 performed a comprehensive review focusing on procedural treatments for acne scars. This review mentioned that there have been a number of procedures for improving acne scars and the choice for each participant is determined mainly by the type of scars present. A combination of procedures is usually required to achieve the best result. The authors stated that fractional laser resurfacing has become a powerful tool in the treatment of acne scars, especially when combined with other treatment methods such as subcision, the chemical reconstruction of skin scars (CROSS) chemical peeling technique with TCA, punch excision or fillers. This conclusion is in disagreement with our review, which failed to find sufficient evidence from RCTs to support fractional laser therapy either alone or combined with punch elevation or PRP for acne scars.
Sobanko 2012 produced a comparative review of laser surgical approaches in the management of acne scarring. They stated that atrophic scars have been best treated with ablative and fractionally ablative and non‐ablative laser systems, depending on individual patient circumstances. These lasers have a role in remodeling the scar contour through neocollagenesis. Non‐ablative laser systems, being less clinically efficacious, may be used in patients asking for a treatment with minimal to no postoperative down time. In recent years, fractional laser scar revision has spanned ablative and non‐ablative laser technologies. It is difficult to provide strong recommendations from our review because the RCT data on fractional laser for acne scars are often limited in terms of number of studies, study size and quality.
Levy 2012 presented a comparative review focusing on the various non‐laser‐based minimally invasive approaches for the treatment of acne scarring. They mentioned superficial chemical peels as a powerful tool in treating atrophic scars with few adverse effects. The efficacy of various treatment methods such as dermabrasion, tissue augmentation, and punch excision has been highlighted focusing on choosing the correct modalities for individual scar types. This conclusion is in agreement with our review, which found moderate‐quality RCT evidence to support injectable fillers in treating atrophic acne scars.
Ong 2012 conducted a review to investigate the effectiveness of ablative and non‐ablative fractional photothermolysis (FP) lasers for treating facial acne scars, and stated that FP technology seemed to improve acne scarring. They concluded that FP technology might be helpful in daily practice for the treatment of acne scars, but found significant limitations comparing published articles on the subject, and no meta‐analyses were possible. Like our review, Ong 2012 was faced by the variability in study parameters, the different subjective improvement rating scales used across the studies, the short‐term reporting of acne scar improvement that could be unreliable, and by the lack of RCTs. Ong 2012 just described an improvement range of 26% to 83% and of 26% to 50% following ablative and non‐ablative FP respectively. Ong 2012 also reported the adverse events associated with FP technology such as it being an uncomfortable procedure and with long‐lasting erythema. Also post‐inflammatory hyperpigmentation is at higher incidence in ablative FP laser compared to non‐ablative FP lasers.
Authors' conclusions
Implications for practice.
Overall, our review found that knowledge gaps predominate over robust evidence for the treatment of acne scars. Only 24 RCTs met our inclusion criteria, with a total of 789 participants. Imprecision due to small numbers of participants led us to downgrade the quality of evidence for several of our comparisons. The small numbers of participants in many studies are not easily remedied due to the fact that most studies are unfunded or minimally funded. In the context that most interventions were investigated by a single RCT, it is difficult to draw meaningful conclusions about efficacy and adverse effects, particularly long‐term effects or delayed events.
The results of this review do not provide sufficient evidence to support the first‐line use of any intervention in the treatment of acne scars, and the relative safety of the different interventions has not been adequately determined.
There is moderate‐quality evidence that injectable fillers improve atrophic acne scars; however, the impermanence of their effect and their minimal utility for fine sharply‐depressed scars (e.g. box‐car and ice‐pick scars) from the clinical point of view should be also considered. Use of non‐fractional non‐ablative laser, fractional laser, fractional radiofrequency, chemical peeling, skin needling, and combined needling with chemical peeling in acne scars is given only limited support, based on evidence from our review.
There is no high‐quality evidence to determine the effects or the safety of subcision, combined fractional laser with PRP, combined fractional laser with punch elevation, and combined microdermabrasion plus ALA‐PDT in acne scars.
There are no RCTs to determine which should be the gold standard treatment against which other treatments should be measured. The utility of full‐face treatment methods for treating atrophic acne scars, whether by laser ablation and thermal destruction (e.g. fully ablative CO₂ resurfacing), mechanical means (e.g. dermabrasion), or chemical dissolution (e.g. phenol peel) have fallen into disfavour due to the attendant protracted post‐treatment down time and relatively elevated risk of long‐term adverse effects, such as scar or hypopigmentation.
The nine studies in ‘Studies awaiting classification’ may alter the conclusions of the review when they are assessed in future updates of this review.
Implications for research.
Our review has highlighted a need for further RCTs to improve the evidence base for most interventions in acne scars. See Table 9 in which we have identified a lack of a validated standardised improvement scale for all the comparisons listed.
Study design
Within‐individual studies may be considered more appropriate than parallel‐group studies, as they reduce the between‐person variance present in parallel studies. Active acne leading to new scar formation may interfere with trials of any design, as does the tendency of some scars to improve spontaneously over time. However some institutional review boards (notably in the USA) find within‐individual studies trials objectionable on ethical grounds, and recruitment into such trials is also difficult.
Trials should include a power calculation and recruit sufficient participants to avoid problems with imprecision due to being underpowered.
Population
Studies should include adults with atrophic or hypertrophic acne scars subdivided by severity and individual scar type. Populations with acne scars on the back should be included. Future trials should collect baseline variables (participant demographics, acne lesions and extent, skin phototype, scar duration, and depth of scars) to ensure that they are balanced.
Intervention
Future studies should include active interventions such as: fractional laser, non‐fractional non‐ablative laser, radiofrequency, microdermabrasion, needling, subcision, punch excision, chemical peeling, injectable fillers, autologous bone marrow stem‐cell transplant, or combined therapy versus placebo or no treatment for atrophic acne scars. For hypertrophic acne scars, future studies should include: Intralesional steroid, low‐level light therapy, cryotherapy, pulsed dye laser, silicone gel, imiquimod, 5‐fluorouracil, interferon, bleomycin, surgery, or combined therapy versus placebo or no treatment.
Recommended comparisons should include investigation of the following benefits or disadvantages:
fractional laser (which has fewer side effects) versus non‐fractional ablative laser (which has more side effects)
fractional laser (as an expensive tool) versus needling or chemical peeling (as economic tools)
fractional laser versus microdermabrasion or versus injectable fillers
fractional laser (which has fewer side effects) versus subcision or punch excision (which has more side effects)
chemical peeling versus needling or injectable fillers or microdermabrasion
needling versus microdermabrasion or subcision or punch excision or fillers
injectable fillers versus autologous bone marrow stem‐cell transplants
low‐level light therapy versus pulsed dye laser or cryotherapy (as an economic tool)
Intralesional steroid (as an economic and practical tool) versus pulsed dye laser, low‐level light therapy or 5‐fluorouracil or bleomycin
Blinding
Participants, clinical investigators and outcome assessors should be blinded to the treatment allocated.
Outcomes
The outcomes of a trial should be prospectively declared in a clinical trial database, including the nature and timing of the primary outcome. Outcomes must include a validated standardised improvement scale assessed by both participants and investigators in the short term (in about six months) and in the long term (at least one year), to determine the treatment effect. Outcomes should also include adverse events serious or severe enough to have caused participants’ withdrawal from the study, as well as less serious adverse events reported by both participants and investigators in the short term (within one month) and in the long term (within six months). Equally important are participant satisfaction, and an assessment of quality of life, as well as a measure of post‐procedure down time assessed in days.
Acknowledgements
We would like to thank Mrs Ola ElDib, country consumer manager, Egypt, for checking the review for readability and clarity. She also ensured that the outcomes are relevant to consumers and contributed to the plain language summary. We would like to thank Dr Marwah Anas El Wegoud and Dr. Ahmed Kolkailah for contributing to data analysis and to the plain language summary.
The authors would like to recognize the hard work and dedication of the Editorial Base, Cochrane Dermatology editor, external referee, Statistics and Methods editors, and last but not least the consumer. Without their sincere support and critical insight, we could not do what we do.
We need to thank the NIHR Health Technology Assessment Programme for making some funding available to complete this previously registered review.
The Cochrane Skin Group editorial base wishes to thank Hywel Williams, who was the Cochrane Dermatology Editor for this review; Matthew Grainge and Sally Wilkes, who were the statistical editors; Esther van Zuuren, who was the methods editor; the clinical referee, Murad Alam; and the consumer referee, Lyn Charland.
Some parts of this review uses text that was originally published in other Cochrane reviews (predominantly Ingram 2015 and Nankervis 2015)
Appendices
Appendix 1. Glossary of medical terms in plain language
| Medical term | Explanation |
| Ablative laser | A laser that removes thin layers of the skin |
| Acellular | Tissue that is not made of cells |
| Atrophic | Loss of tissue |
| Blackhead comedones | Comedones (primary lesion for acne) that are open at the surface of the skin and filled with excess oil and dead skin cells |
| Chronic | Persisting for a long time or constantly recurring |
| Collagen | Extracellular fibres arranged in bundles representing the major component of the deeper layer of the skin |
| Comedone | Primary lesion diagnostic for acne disorder whether blackhead or whitehead |
| Dermis/dermal | Deep layer of the skin |
| Epidermis/epidermal | Superficial layer of the skin |
| Erythema | Redness |
| Fraise | A diamond wheel with rough edges used to remove the upper layers of the skin |
| Hyalinised | Transformation of a substance to a glasslike or transparent state |
| Hyperpigmentation | Increased pigmentation |
| Hyperproliferation | Abnormally high rate of proliferation |
| Hypertrophic | Abnormal enlargement of a part or organ |
| Intralesional | Injection inside the lesion itself |
| Keloids | Hard growths as scar tissue grows excessively |
| Microneedling | The use of small needles to cause punctures in the skin for therapeutic purposes |
| Milia | A small white or yellowish nodule produced in the skin by the retention of sebaceous secretion |
| Morphology | Shape |
| Nodules | Solid elevation in the skin measuring 0.5 cm or more in diameter |
| Nodulocystic | Having both nodules and cystic lesions |
| Oedema | An accumulation of an excessive amount of watery fluid in tissues |
| Papules | Solid elevation in the skin measuring less than < 0.5 cm in diameter |
| Pilosebaceous unit | Epidermal structure containing both hair follicle and sebaceous gland |
| Pustules | Small elevation of the skin containing pus |
| Re‐epithelialize | Restoration of structure of injured tissue |
| Subcision | A process through which you separate the skin tissue from the deeper scar tissue |
| Whitehead comedones | Comedones (primary lesion for acne) that stay closed at the surface of the skin when oil and skin cells prevent a clogged hair follicle from opening. |
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor: [Acne Vulgaris] explode all trees #2 acne:ti,ab,kw #3 #1 or #2 #4 MeSH descriptor: [Cicatrix] explode all trees #5 (scar or scars or scarred or scarring or scarification):ti,ab,kw #6 cicatri*:ti,ab,kw #7 #4 or #5 or #6 #8 #3 and #7
Appendix 3. MEDLINE (Ovid) search strategy
1. exp Cicatrix/ 2. cicatri$.ti,ab. 3. scarring.ti,ab. 4. scar$1.ti,ab. 5. scarification.ti,ab. 6. scarred.ti,ab. 7. or/1‐6 8. exp Acne Vulgaris/ 9. acne.ti,ab. 10. 8 or 9 11. randomised controlled trial.pt. 12. controlled clinical trial.pt. 13. randomized.ab. 14. placebo.ab. 15. clinical trials as topic.sh. 16. randomly.ab. 17. trial.ti. 18. 11 or 12 or 13 or 14 or 15 or 16 or 17 19. (animals not (humans and animals)).sh. 20. 18 not 19 21. 7 and 10 22. 20 and 21
[11‐20: Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision)]
Appendix 4. Embase (Ovid) search strategy
1. scar$1.ti,ab. 2. scarred.ti,ab. 3. scarring.ti,ab. 4. exp scar formation/ or exp skin scar/ or exp scar/ 5. scarification.ti,ab. 6. cicatri$.ti,ab. 7. or/1‐6 8. exp acne vulgaris/ or exp acne/ 9. acne.ti,ab. 10. or/8‐9 11. 7 and 10 12. crossover procedure.sh. 13. double‐blind procedure.sh. 14. single‐blind procedure.sh. 15. (crossover$ or cross over$).tw. 16. placebo$.tw. 17. (doubl$ adj blind$).tw. 18. allocat$.tw. 19. trial.ti. 20. randomised controlled trial.sh. 21. random$.tw. 22. or/12‐21 23. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 24. human/ or normal human/ 25. 23 and 24 26. 23 not 25 27. 22 not 26 28. 11 and 27
Appendix 5. LILACS search strategy
acne and (scar$ or cicatri$ or escarificacion) and the Controlled clinical trials topic‐specific query filter.
Data and analyses
Comparison 1. Non‐fractional non‐ablative laser versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Within‐individual studies | Other data | No numeric data |
Comparison 2. Fractional laser versus non‐fractional non‐ablative laser.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant‐reported scar improvement (short‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Investigator‐assessed scar improvement (short‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Participant‐reported adverse events (short‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Burning | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Investigator‐assessed adverse events (short‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Hyperpigmentation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Fractional laser versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Within‐individual studies | Other data | No numeric data |
Comparison 4. Fractional laser versus radiofrequency.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant‐reported scar improvement (short‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Investigator‐assessed scar improvement (short‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Within‐individual studies | Other data | No numeric data |
Comparison 5. Fractional laser versus combined fractional laser plus any active intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Within‐individual studies | Other data | No numeric data |
Comparison 6. Fractional laser versus chemical peeling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Within‐individual studies | Other data | No numeric data |
Comparison 7. Fractional laser versus combined chemical peeling plus needling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant‐reported scar improvement (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Investigator‐assessed scar improvement (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Participant‐reported adverse events (< 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 8. Chemical peeling versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious or severe adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 9. Chemical peeling versus combined chemical peeling plus any active intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant‐reported scar improvement (8 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Investigator‐assessed scar improvement (8 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Participant‐reported adverse events (< 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Investigator‐assessed adverse events (8 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 10. Chemical peeling versus needling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant‐reported scar improvement (1 month) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Investigator‐assessed scar improvement (1 month) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Patient satisfaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Participant‐reported adverse events (< 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Investigator‐assessed adverse events (1 month) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Hyperpigmentation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Post‐procedure down time | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 11. Needling versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Within‐individual studies | Other data | No numeric data |
Comparison 12. Injectable fillers versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant‐reported scar improvement (short‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Investigator‐assessed scar improvement (short‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Participant satisfaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Participant‐reported adverse events (short‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Investigator‐assessed adverse events (short‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Within‐individual studies | Other data | No numeric data |
Comparison 13. Injectable fillers versus subcision.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Within‐individual studies | Other data | No numeric data |
Comparison 14. Combined microdermabrasion plus ALA‐PDT versus combined microdermabrasion plus placebo‐PDT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Within‐individual studies | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alam 2014.
| Methods | This pilot study was a single‐centre (at an urban academic institution), rater‐blinded, split‐face, placebo‐controlled, randomised clinical trial (from 30‐November‐2009 through 27‐July‐2010). | |
| Participants | 20 healthy adults (both genders) were enrolled (5 individuals dropped out, 15 completed the treatment and are analysed) Inclusion: "Age of 18 to 70 years, good general health, Global Acne Scarring Classification grades 2 through 4, and at least 2 5 × 5‐cm areas of acne scarring on the face (with at least 3 definable acne scars in each area)". Exclusion: "History of keloids or hypertrophic scars, skin infection or active skin disease other than mild acne in or around the study areas, active systemic or local skin disease likely to alter wound healing, treatment within the last 6 months or pending treatment within the subsequent 6 months with injectable fillers or ablative or non‐ablative laser resurfacing to the study areas, medication with isotretinoin or other oral retinoids within the past 12 months, current treatment with anticoagulants or antithrombotics, or allergy to topical anaesthetics". |
|
| Interventions | Intervention: 3 treatment visits were performed at 2‐week intervals (i.e. weeks 1, 3, and 5). At each of these visits, needling was performed on the study treatment area. Comparator: 3 treatment visits were performed at 2‐week intervals (i.e. weeks 1, 3, and 5). At each of these visits, topical anaesthetic was only massaged into the control area. |
|
| Outcomes | Improvement of acne scars (6 months), using the quantitative global scarring grading system, developed by Goodman 2006b then a scale of 1 to 4 (1 = < 25% improvement; 2 = 26% ‐ 50%; 3 = 51% ‐ 75%; 4 = > 75%) Participant satisfaction (6 months), using a word scale (very satisfied, satisfied, slightly satisfied, and unsatisfied) Any adverse event (6 months) Timing: at baseline, at 3 months and 6 months |
|
| Funding source | Departmental Research Funds, Department of Dermatology, Northwestern University, Chicago, Illinois | |
| Declaration of interest | Dr Alam is employed at Northwestern University. Northwestern University has a clinical trials unit that receives grants from corporate and governmental entities to perform clinical research. Dr Alam has been a consultant for Amway and Leo Pharma, both unrelated to this research. Dr Alam has been principal investigator on studies funded in part by Allergan, Medicis, Bioform, and Ulthera. In all cases, grants and gifts in kind have been provided to Northwestern University and not Dr Alam directly, and Dr Alam has not received any salary support from these grants. Dr Alam receives royalties of less than USD 5000 per year from Elsevier for technical books he has edited. | |
| Notes | USA Approved by the Northwestern University Institutional Review Board The trial was registered as ClinicalTrials.gov Identifier: NCT00974870 Written informed consent was obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly generated 1’s and 2’s were used to assign the leftmost labelled acne scar area on a given participant to the treatment arm (1) or the control arm (2), with the contralateral side then receiving the remaining assignment." Comment: Insufficient data |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomly generated 1’s and 2’s were sealed separately in opaque envelopes." Comment: Probably done. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Given that the control arm was easily distinguished from the treatment arm during treatment, participants and the treating dermatologist were not blinded." Comment: Probably not done. |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "The two dermatologist raters of photographs did not participate in randomisation or treatment and therefore were able to be blinded". Comment: Probably done. |
| Blinding of outcome assessment (detection bias) Participant‐reported | High risk | Quote: "Given that the control arm was easily distinguished from the treatment arm during treatment, participants and the treating dermatologist were not blinded." Comment: No blinding of participants and the outcome assessment is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 individuals consented, and 5 dropped out before the first treatment. The remaining 15 completed all treatments and are analysed. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s prespecified outcomes that are of interest in the review have been reported in the prespecified way |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Asilian 2011.
| Methods | A randomised blinded clinical trial (from 01‐March‐2009 through 01‐October‐2010) | |
| Participants | 64 participants (skin type II IV, aged 19 43 years) presenting with moderate to severe atrophic facial acne scars Inclusion: "Any type of moderate to severe facial atrophic acne scar (rolling, boxcar, ice pick)" Exclusion: "Pregnancy, lactation, history of keloid formation, immunosuppressant or isotretinoin use, and filler substance injections or skin resurfacing by dermabrasion or lasers within the preceding 6 months" |
|
| Interventions | Intervention: 32 participants received QSwitched 1064nm Nd: YAG (Venus 3, Input Voltage 22v/50Hz, April 2003, Korea) for the entire face by a single operator with an average fluence of 2.5 J/cm, spot size: 7 mm for a total of 4 treatments at 4week intervals Comparator: 32 participants received fractional CO₂ laser (Pixel Alma 10600nm) for the entire face by a single operator using pulse width of 110 msec (ontime), 600 msec (offtime) and pulse duration of 350 μs for a total of 4 treatments at 4week intervals |
|
| Outcomes | Improvement of acne scars (6 months), using a quartile grading scale: mild = < 25%; moderate = 25% ‐ 50%; good = 51% ‐ 75%; excellent = 76% to 100% Participant satisfaction (6 months), using satisfaction survey: mild = < 25%; moderate = 25% ‐ 50%; good = 51% ‐ 75%; excellent = 76% to 100% Any adverse event (3 weeks) Timing: 3 and 6 months after the 4th session of treatment |
|
| Funding source | No available data | |
| Declaration of interest | Authors have no conflict of interests | |
| Notes | Iran The study was approved by the ethical committee of Isfahan University of Medical Science Written informed consent was obtained from all participants before enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were divided into two different treatment groups, using a table of random numbers." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment process to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "Assessments of the treatment areas using comparative photographs were performed by two blinded dermatologists" Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 64 participants completed 4 treatment sessions and all of them were followed up for 6 months after the last session |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Bernstein 2001.
| Methods | A pilot split‐face study to explore the side‐effect profile and subjective improvement of acne scarring afforded by non‐ablative resurfacing. The treatment side was randomised from participant to participant | |
| Participants | 11 women (between the ages of 43 and 64 years and with Fitzpatrick skin types I through III) with acne scarring. Inclusion: women with mild‐to‐moderate acne scarring Exclusion: deep ice‐pick scars or previously treated with laser resurfacing |
|
| Interventions | Intervention: A frequency‐doubled Nd:YAG laser was used on 1 cheek at a wavelength of 532nm, a spot diameter of 3 mm, and a pulse duration of 2 ms (VersaPulse‐C, Coherent Medical Lasers, Santa Clara, CA, USA). The laser was used for 2 ‐ 4 sessions with time interval between treatment sessions ranging from 3 to 6 weeks Comparator: The contra‐lateral side was kept as an untreated control. |
|
| Outcomes | improvement of acne scars (by observer) (6 months) improvement of acne scars (by participant) (3 months), using a percentage improvement over their pretreatment condition Side effects (1 week) Timing: 1 week, 3 months, and 6 months |
|
| Funding source | No available data | |
| Declaration of interest | No available data | |
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomised to receive treatment on one cheek, while the contra‐lateral side was kept as an untreated control." Comment: Insufficient data |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Subjects with acne scarring were randomized to receive treatment on one cheek, while the contra‐lateral side was kept as an untreated control." Comment: Given that the control arm was kept untreated, participants and the treating dermatologist were not blinded |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "An observer blinded as to which side received treatment attempted to identify the treated side 6 weeks following the final treatment." Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | High risk | Quote: "Subjects with acne scarring were randomized to receive treatment on one cheek, while the contra‐lateral side was kept as an untreated control." Comment: No blinding of participants and the outcome assessment is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and were analysed |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Chae 2015.
| Methods | A randomised, controlled, single‐blinded study (from 01‐September‐2012 through 01‐March‐2013) | |
| Participants | 40 participants with atrophic acne scars Inclusion: Healthy, with no dermatologic or any other disorder, except for acne scars Exclusion: Patients who had received acne scar treatment during the prior 6 months, pregnant or lactating |
|
| Interventions | Intervention: Group A (n = 20) received the 1550 nm Er:Glass fractional laser (FXL) with a Sellas apparatus (Dinona, Daejeon, Korea) at 4‐week intervals. FXL was performed on the basis of 500 MTZ/cm2 and 15–20 mJ/MTZ energy level Comparator; Group B (n = 20) received the fractional radiofrequency microneedle (FRM) utilising the Inskin device (Einsmed, Seongnam, Korea) at an intensity of 40 – 60 W (maximum power 80 W, 2‐mm‐depth needle with 36 microneedle electrode tip) and 0.1 ms radiofrequency conduction time in the continuous wave mode. |
|
| Outcomes | Improvement of acne scars (8 weeks), using a 5‐point scale (1 = no change, 0%; 2 = slight, 0% – 25%; 3 = average, 26% – 50%; 4 = good, 51% – 75%; 5 = excellent, 76% – 100%) Participant satisfaction (8 weeks), using 5‐point scale of self‐assessed participant satisfaction (1 = no change, 0%; 2 = slight, 0% – 25%; 3 = average, 26% – 50%; 4 = good, 51% – 75%; 5 = excellent, 76% – 100%) Side effects (8 weeks) Timing: week 4, week 8, week 12, and week 20 |
|
| Funding source | No available data | |
| Declaration of interest | No available data | |
| Notes | Korea Approved by the Institutional Review Board, before enrolment. All participants provided written informed consent. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly and equally distributed into either group A (FXL) or group B (FRM)." Comment: Insufficient data |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "single‐blinded study" Comment: Probably not done |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "Two independent dermatologists not involved in the study examined the serial photos and rated the overall improvements compared to baseline." Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | High risk | Quote: "single‐blinded study" Comment: No blinding of participants and the outcome assessment is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 40 participants completed the study and were analysed |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Cho 2010.
| Methods | A randomised, split‐face, evaluator‐blinded study | |
| Participants | 8 men (mean age 21.3 years, range 20 – 23; Fitzpatrick skin type IV), with mild‐to‐severe atrophic acne scars Exclusion: "Patients who had undergone concomitant treatments including skin resurfacing procedures, chemical reconstruction of skin scars (CROSS) using trichloroacetic acid, collagen induction therapy using a micro‐needle therapy system, non‐ablative fractional photothermolysis system and CO₂ fractional laser treatments within the previous 6 months. Patients with keloids, pregnancy, immunosuppression and history of isotretinoin." |
|
| Interventions | Intervention: 1 side of each participant’s face was treated with a single session of non‐ablative 1550‐nm erbium‐doped fractional photothermolysis systems (FPS) using the Fraxel SR1500 (Reliant Technologies, Mountain View, CA, USA) Comparator: The other side of the face was treated with a single session of CO₂ fractional laser systems (CO₂ FS) using the 10600‐nm Ultrapulse Encore laser (Lumenis Inc., Santa Clara, CA, USA) |
|
| Outcomes | Improvement of acne scars (3 months), using quartile grading scale for evaluations: grade 1 = < 25%, minimal to no improvement; grade 2 = 26% ‐ 50%, moderate improvement; grade 3 = 51% ‐ 75%, marked improvement; grade 4 = > 75%, near‐total improvement Participant satisfaction (3 months), using the following scale: very satisfied, satisfied, slightly satisfied and unsatisfied Side effects (3 months) Timing: at baseline and 3 months after the treatment |
|
| Funding source | No available data | |
| Declaration of interest | Nothing to be declared | |
| Notes | Korea Approved by the institutional review board of Bundang CHA General Hospital, Pochon CHA University, College of Medicine, Seongnam |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A randomised, split‐face, evaluator‐blinded study" Comment: Insufficient data |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "Objective clinical assessments were accomplished separately for each side of the face by two blinded dermatologists in non‐chronological order" Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed treatment sessions and all were analysed |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Erbağci 2000.
| Methods | A single, blind, placebo‐controlled, randomised comparative clinical study. | |
| Participants | 58 women, ranging in age from 18 ‐ 41 years, with atrophic acne scars Exclusion: "Hypertrophic, depressed‐fibrotic, and ice‐pick scars or keloids and those with severe active inflammatory acne lesions, pregnancy, lactation, a history of isotretinoin ingestion in the preceding 6 months, concomitant use of an oral contraceptive or any hormone preparation, the presence of active herpes infection, concomitant serious systemic or skin disease, depression and antidepressive therapy, and a history of hypertrophic scar or keloid". |
|
| Interventions | 23 participants received biweekly serial glycolic acid (GA) peels in a gradually increasing manner in time (2 ‐ 5 mins) and concentration (20%, 35%, 50%, 70% GA) 20 participants were instructed to use 15% GA home‐care product twice daily for a period of 24 weeks 15 participants received a base cream for 24 weeks |
|
| Outcomes | Improvement of acne scars (6 months), using a 10‐point scale as follows: 0 = No scar; 1 = very mild; 2 ‐ 3 = mild; 4 ‐ 7 = moderate; 8 ‐ 9 = severe; 10 = very severe Side effects (6 months) Timing: at baseline, at 4‐week intervals, and at 6 months |
|
| Funding source | No available data | |
| Declaration of interest | No available data | |
| Notes | Turkey Participants gave informed consent before enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly divided into three groups." Comment: Insufficient data |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: " A single, blind, ...." Comment: No blinding of study participants and personnel |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "Clinical assessments were conducted by an independent blind investigator" Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | High risk | Quote: " A single, blind, ...." Comment: No blinding of participants and the outcome assessment is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 48/58 participants (16 in group A, 18 in group B, 14 in group C) completed the study. 7 women from group A withdrew because they were unable to tolerate concentrations > 20% or 35% and contact times > 2 mins. 3 women (2 from group B and 1 from group C) were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Faghihi 2015.
| Methods | A randomised splitface clinical trial | |
| Participants | 42 Iranian patients aged 18 – 55 years Inclusion: "Fitzpatrick skin types III to IV and moderate to severe atrophic acne scars on both cheeks" Exclusion: "Pregnancy, lactation, active inflammatory acne, Immunocompetence, history of deep chemical peeling or filler injection in the previous 6 months, history of hypertrophic scars and keloids, use of isotretinoin in the previous 6 months, allergy to anaesthesia, active infection in the treatment area, pre‐malignant or malignant lesions in the treatment area, bleeding tendencies, and history of herpes simplex or herpes zoster infection on the face". |
|
| Interventions | Intervention: 1 side of the participant's face was treated using the 10600nm fractional CO₂ laser alone (M×7000/Stamp Type, Daeshin, South Korea) Comparator: The other side of the face was treated with the same fractional CO₂ laser plus punch elevation (2.5 – 3 mm biopsy disposable punches) |
|
| Outcomes | Improvement of acne scars (4 months), using a grading scale as follows: 1 = < 25% (minimal) improvement; 2 = 25% – 50% (moderate) improvement; 3 = 51% – 75% (good) improvement; 4 = > 75% (excellent) improvement Participant satisfaction (4 months), using a VAS; (rating of 0 was no satisfaction, and a rating of 10 was the best possible satisfaction) Side effects (4 months) Timing: 1 and 4 months after the second treatment session |
|
| Funding source | Skin Diseases and Leishmaniasis Research Center, Isfahan University of Medical Sciences, Isfahan, Iran | |
| Declaration of interest | Nothing to be declared | |
| Notes | Iran The Isfahan University of Medical Sciences Ethical Committee, Isfahan, Iran, approved the study protocol. The trial was registered in the Iranian Registry of Clinical Trials: IRCT2014080218647N1. Note: trial was registered after the start of the trial Participants signed an informed consent form for participation in the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using random allocation software" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "The assignment sequence was concealed in opaque envelopes." Comment: Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "One side received fractional CO laser treatment and the other received one session of punch elevation combined with two sessions of laser fractional CO laser treatment" Comment: Probably not done. Given that the control arm was easily distinguished from the treatment arm during treatment, participants and the treating dermatologist were not blinded |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "Two dermatologists blinded to treatment side evaluated clinical improvement of acne scars." Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | High risk | Quote: "One side received fractional CO laser treatment and the other received one session of punch elevation combined with two sessions of laser fractional CO laser treatment" Comment: No blinding of participants and the outcome assessment is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 42 participants (100%) completed the 2 treatment sessions, and all were followed up for 4 months after the last treatment session |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's prespecified outcomes that are of interest in the review have been reported in the prespecified way |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Hedelund 2010.
| Methods | A split‐face randomised clinical trial with blinded on‐site response evaluations in a hospital setting Duration of the trial is 5 months (treatment duration: 2 months and follow‐up duration: 3 months) (from 01‐November‐2007 through 01‐Jun‐2008) |
|
| Participants | 10 participants were enrolled Inclusion: "age 18 – 60 years, white, with skin type I – IV, duration of atrophic acne scars 1 year or more, scarring manifested such that 2 areas of similar size and appearance were available in contralateral anatomical regions (cheeks, temporal regions, forehead, back), and willingness and ability to comply with the requirements of the protocol". Exclusion: "a tendency to produce hypertrophic scars or keloids, previous treatment of the study areas with dermabrasion, chemical peeling, filler, laser treatment or intense pulsed light, photosensitivity, pregnancy or lactation, current treatment with anticoagulative medication, treatment with oral retinoid drugs within the past 6 months, pigmentation after recent exposure to the sun or use of a solarium, and potential inability to follow the treatment protocol". |
|
| Interventions | Intervention: An area on the site (A) received 3 active erbium:glass rod laser treatment sessions at 4‐week intervals using a StarLux‐300 with a Lux 1,540‐nm fractional handpiece (Palomar Medical Technologies, Burlington, MA). The same physician performed all treatments (K.M.) Comparator: The area on the contralateral site (B) received no treatment |
|
| Outcomes | Improvement in scar texture (12 weeks), using a numerical scale ranging from 0 to 10 (0 even skin texture without scarring, 5 moderate scarring, and 10 worst possible scarring)
Side effects (12 weeks)
Participant satisfaction (12 weeks), as follows: 0 no satisfaction, 10 best imaginable satisfaction
Overall acne scar appearance (12 weeks), using a 5‐point scale (worse, not improved, slightly improved, moderately improved, significantly improved) Timing: at week 4 and week 12 after the final treatment |
|
| Funding source | MediTech, Scandinavia, Denmark provided on loan a StarLux 1540 nm fractionated handpiece, had no role in the design or conduct of the study, in the collection, analysis and interpretation of the data, or in the preparation, review and approval of the manuscript | |
| Declaration of interest | Dr. Haedersdal received a research grant and a StarLux 1540 nm fractionated handpiece on loan from MediTech, Scandinavia, Denmark, to support this study. The authors have no relevant personal financial interest in this article | |
| Notes | Denmark Written informed consent was obtained from all study participants The study was approved by the Committee on Biomedical Research Ethics of Copenhagen and Frederiksberg |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before treatment two contralateral areas of similar size and appearance were outlined and marked “A” and “B”. The areas were randomised to treatment or no treatment. Randomization and allocation was carried out by the patient selecting one of two opaque sealed envelopes containing a card with the treatment code (“laser treatment” of site A and “no treatment” of site B)." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "By the patient selecting one of two opaque sealed envelopes containing a card with the treatment code (“laser treatment” of site A and “no treatment” of site B)." Comment: Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The areas were randomized to treatment or no treatment." Comment: Probably not done. Given that the control arm was easily distinguished from the treatment arm during treatment, participants and the treating dermatologist were not blinded |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "...with blinded on‐site response evaluations" "This treating investigator was not included in the assessment of patients before and after treatment. Moreover, patients were instructed not to inform the evaluating physician of which area had been treated and which not treated." Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | High risk | Quote: "The areas were randomized to treatment or no treatment." Comment: No blinding of participants and the outcome assessment is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the allocated treatments and were included in the analyses |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Hedelund 2012.
| Methods | A split‐face randomised clinical trial Duration of study: 8 months (treatment duration: 8 ‐ 10 weeks and follow‐up duration: 6 months) (from 01‐December‐2909 through 01‐November‐2010) |
|
| Participants | 13 healthy volunteers (6 men and 7 women). They had moderate to severe atrophic acne scars (Icepick, rolling and boxcar types). Inclusion: age of 18 – 60 years, white, with skin types I – III, duration of atrophic acne scars 1 year or more, willingness and ability to comply with the requirements of the protocol. Exclusion: History of hypertrophic scars or keloids, "previous treatment with ablative lasers of study areas, photosensitivity, pregnancy or lactation, current anticoagulative medication, oral retinoid drugs within the past 6 months, pigmentation after recent exposure to sun or solarium, and patients not considered to be able to follow the treatment protocol" |
|
| Interventions | Intervention: An area ((9 – 30 cm²) on site A received 3 laser treatments at 4‐ to 5‐week intervals. The laser system was a CO₂ laser (MedArt 610) equipped with a scanner (MedArt 458) developed specifically for fractional treatments (MedArt, Hvidovre, Denmark) Comparator: A similar area on site B received no treatment |
|
| Outcomes | Improvement of scars texture and depth using a numerical scale ranging from 0 (even skin texture without scarring/atrophy) to 10 (worst possible scarring/atrophy). Time frame: 1, 3, and 6 months after the final treatment Participant satisfaction and participant self assessments of scar texture improvement using a numerical scale from 0 (unsatisfied/even skin texture without scarring) to 10 (maximal satisfaction/worst possible scarring). Time frame: 1, 3, and 6 months after the final treatment Pain assessed using a numerical scale ranging from 0 (no pain) to 10 (worst imaginable pain). Time frame: immediately after treatment Adverse effects using a numerical scale ranging from 0 to 3 (0 = absent; 1 = mild; 2 = moderate; 3 = severe). Time frame: at 2 or 3 days after first treatment and 1, 3, and 6 months after the final treatment |
|
| Funding source | A grant by MedArt A/S, Hvidovre, Denmark | |
| Declaration of interest | Nothing to be declared | |
| Notes | Denmark The study was approved by The Ethical Committee of Copenhagen and Frederiksberg Written informed consent was obtained from all study participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Two facial areas of similar size and appearance were outlined, marked ‘‘A’’ and ‘‘B’’ and randomised to treatment versus no treatment. Randomisation by patients drawing lots between opaque sealed envelopes, containing cards with treatment code (‘‘laser treatment’’ of site A and ‘‘no treatment’’ of site B or vice versa)." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients drawing lots between opaque sealed envelopes, containing cards with treatment code" Comment: Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Two facial areas ...... were outlined, marked ‘‘A’’ and ‘‘B’’ and randomized to treatment
versus no treatment" Comment: Probably not done. Given that the control arm was easily distinguished from the treatment arm during treatment, participants and the treating dermatologist were not blinded |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "Treatment response and adverse effects were evaluated by clinical on‐site evaluations by three blinded physicians. The treating investigator was not included in preoperative or post‐treatment assessments of patients. Moreover, patients were instructed not to inform the evaluating physicians of which area was treated or untreated." Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | High risk | Quote: "Two facial areas ...... were outlined, marked ‘‘A’’ and ‘‘B’’ and randomized to treatment
versus no treatment" Comment: No blinding of participants and the outcome assessment is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13 participants were included in the study and received the allocated treatments. 12 completed the study (1 participant withdrew before the final evaluation and was not included in the analysis 6 months postoperatively) |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Karnik 2014.
| Methods | A double‐blind, randomised controlled trial conducted at 10 investigative centres across the USA | |
| Participants | Potential 147 participants with acne scars (average age 44 years, 39% male) Inclusion: 18 years of age or older, at least 4 moderate to severe, atrophic, distensible acne scars of the cheek, willingness to withdraw any confounder therapies Exclusion: "Pregnancy or breastfeeding, prohibited therapies within a time frame which could obscure the results of the study, inflammatory skin diseases including acne (> 3 inflammatory lesions per cheek), history of granulomatous or connective tissue disease, hypertrophic scarring, predominance of acne scars other than rolling scars, allergy to beef, beef products or any components of the study materials" |
|
| Interventions | Intervention: 97 participants received injection with PMMA suspended in bovine collagen (ArteFill, Suneva Medical Inc, Santa Barbara, CA) in a 2:1 fashion Comparator: 50 participants received saline injections in a 2:1 fashion. Control injections were performed with preservative‐free saline in a similar manner |
|
| Outcomes | Improvement of acne scars (6 months) using Physician and Subject Global Aesthetic Improvement Scales Participant satisfaction (6 months) using a scale as follows: 6 = very satisfied; 5 = satisfied; 4 = somewhat satisfied; 3 = somewhat dissatisfied; 2 = dissatisfied; 1 = very dissatisfied Side effects (6 months) |
|
| Funding source | Suneva Medical Inc | |
| Declaration of interest | Nothing to be declared | |
| Notes | USA The trial was registered as ClinicalTrials.gov Identifier: NCT01559922 Before screening, participants underwent an informed consent process and signed an Institutional Review Board approved informed consent form |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomised to receive either PMMA‐collagen or saline injections in a 2:1 fashion respectively, using a randomisation system that controlled for gender and Fitzpatrick skin type. Treatment centres had no access to randomisation data." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment process to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A double‐blind trial. Control injections were performed with preservative‐free saline in a similar manner." Comment: Probably done |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "A blinded investigator who performed subject evaluations only without knowledge of the treatment assignment." Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | Low risk | Qoute: "A double‐blind trial. Control injections were performed with preservative‐free saline in a similar manner." Comment: Probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 199 participants were screened, 175 were randomised, and 147 received at least 1 injection and were included in the primary analysis |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s prespecified outcomes that are of interest in the review have been reported in the prespecified way |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kim 2009.
| Methods | A split‐face trial conducted in patients with acne scars. Facial halves were randomly assigned to receive either treatment | |
| Participants | 20 participants (6 women and 14 men) aged 22 – 37 years with mild to moderate acne scars and Fitzpatrick Skin Types IV and V. The participants were grouped as rolling type group and icepick type group according to the predominant scar type Exclusion: "Pregnancy or lactation, history of hypertrophic scarring or keloid formation, history of active or recurrent herpes simplex, presence of infected skin lesions, refusal to give signed informed consent, and use of isotretinoin within 6 months before treatment" |
|
| Interventions | Intervention: 1 facial half received treatment with 1550 nm Er:Glass fractional laser (Mosaic1, Lutronic Corporation, Gyeonggi, Korea). The 1550 nm Er:Glass fractional laser applied pulse energy of 30 – 32 mJ and density of 300 – 350 spots/cm2 using a 6 mm handpiece tip 3 times during a 6‐week interval Comparator: The contralateral half received CROSS method using 100% TCA. The CROSS method repeated 2 times every 12 weeks |
|
| Outcomes | Improvement of acne scars (12 weeks), using a quartile scale (0 = no improvement; 1 = 1% – 25% improvement; 2 = 26% – 50% improvement; 3 = 51% – 75% improvement; 4 = > 75% improvement) Participant satisfaction (12 weeks) using the same quartile scale Side effects (12 weeks), including severity of the pain on a 10‐point scale (0 – 9), erythema lasting days, and overall down time Timing: at baseline, at week 6, week 12, week 18, week 24,and 12 weeks after the final treatment |
|
| Funding source | Yonsei University Research Fund of 2007 | |
| Declaration of interest | No available data | |
| Notes | Korea Informed consent was obtained from all participants Study approved by the hospital’s medical ethnic committee |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Facial halves were randomly assigned" Comment: Insufficient data |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment process to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "One side was treated with the 1,550nm Er:Glass fractional laser .....And the other side was treated with CROSS method " Comment: Probably not done. Given that the control arm was easily distinguished from the treatment arm during treatment, participants and the treating dermatologist were not blinded |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "Patient photographs were reviewed by 2 independent physicians who were blinded to the study." Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | High risk | Quote: "One side was treated with the 1,550nm Er:Glass fractional laser .....And the other side was treated with CROSS method " Comment: No blinding of participants and the outcome assessment is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18/20 participants completed the trial and were included in the data. 1 participant dropped out because of slight discomfort of the treatment such as pain and erythema, and the other dropped out because of scheduling conflicts |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Lee 2009.
| Methods | A 14‐week, single‐blinded, randomised, comparative split‐face study of a 585‐nm PDL and a 1064‐nm long‐pulsed Nd:YAG laser for the treatment of atrophic facial acne scars | |
| Participants | 18 participants (10 men, 8 women) Inclusion: "Age of at least 18 years and a diagnosis of mild to moderate atrophic acne scarring" Exclusion: "Known photosensitivity, pregnancy or lactation, a history of hypertrophic or keloidal scarring, the use of isotretinoin, a history of facial laser treatment or surgical procedure within 6 months of study enrolment, and patients with a medical condition that might have influenced the wound healing process" |
|
| Interventions | Intervention: 1 side of the face was treated with non‐overlapping pulses of 585‐nm PDL (Cynergy, Cynosure Inc, Westford, MA) at a sub‐purpuric fluence of 10 to 11 J/cm2 and a 40‐ms pulse duration using a 7‐mm hand piece. All participants received 4 treatment sessions at 2‐week intervals Comparator: At the same session, the contralateral side was treated with a 1064‐nm long‐pulsed Nd:YAG laser (Cynergy) at a fluence of 50 to 70 J/cm² and a 50‐ to 100‐ms pulse duration using a 7‐mm spot size All participants received 4 treatment sessions at 2‐week intervals |
|
| Outcomes | Improvement of acne scars (14 weeks), using percentage improvements (0% ‐ 100%) versus baseline Patient satisfaction (14 weeks), using a scale of 0 (neutral) to 10 (highly satisfied) Side effects (14 weeks) Timing: at week 2, week 4, week 6, week 10, week 14 |
|
| Funding source | No available data | |
| Declaration of interest | No available data | |
| Notes | Korea Study approved by the Institutional Review Board of Seoul National University Hospital Written informed consent was obtained from all participants before enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At treatment sessions, according to baseline randomization, affected areas on one side of the face were treated with one type of laser. Similarly, at these sessions, contralateral sides were treated with the other laser type." Comment: Insufficient data |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment process to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "single blinded" Comment: Probably not done |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "Acne scar improvements were quantified by assessing the degrees of improvement by two masked dermatologists." Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | High risk | Quote: "single blinded" Comment: No blinding of participants and the outcome assessment is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 participants enrolled in this study and completed the 14‐week study |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Lee 2011.
| Methods | A split‐face randomised trial. Each participant’s entire face was then treated with an ablative CO₂ fractional laser (Q‐ray, Diosis Inc., Seoul, Korea) using a pulse energy of 25 mJ per fixed 150‐mm‐diameter microbeam and a density of 400MTZ/cm² | |
| Participants | 14 Korean participants (Fitzpatrick skin types III – V) with moderate to severe acne scars. The mean participant age was 28.1 (range 21 – 38), and the sample included 4 women and 10 men Exclusion: "History of keloid scar formation, any active inflammation, oral isotretinoin use within the preceding 6 months, diabetes, collagen vascular disease, or ablative or non‐ablative laser skin resurfacing within the preceding 12 months, pregnant or lactating women. Formation, any active inflammation, oral isotretinoin general vascular disease, or ablative or non‐ablative laser skin resurfacing within the preceding 12 months" |
|
| Interventions | Intervention: After ablative CO₂ laser resurfacing, 1 facial half received intradermal treatment with 0.3 mL autologous PRP at 20 individual sites. Sites were spaced at 1.5 to 2 cm intervals. 1 month after the initial treatment, all participants underwent 1 additional treatment session with the same therapeutic protocol. Comparator: After ablative CO₂ laser resurfacing, the other facial half received intradermal injection with 0.3 ml normal saline at 20 individual sites spaced at 1.5 to 2 cm intervals. 1 month after the initial treatment, all participants underwent 1 additional treatment session with the same therapeutic protocol |
|
| Outcomes | Improvement of acne scars using a quartile grading scale (0 = no improvement; 1 = < 25% improvement; 2 = 25% – 50% improvement; 3 = 51% – 75% improvement; 4 = > 75% improvement). Timing: baseline and 4 months after the last treatment Side effects: Erythema and oedema were graded on a 5‐point scale (0 = none; 1 = trace; 2 = mild; 3 = moderate; 4 = severe).Timing: days 0, 2, 4, 6, 8, 15, and 30 |
|
| Funding source | Chung‐Ang University | |
| Declaration of interest | The authors have indicated no significant interest with commercial supporters | |
| Notes | South Korea | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Facial halves were randomly assigned" Comment: Insufficient data |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment process to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "Two different blinded dermatologists evaluated overall clinical improvement, comparing digital photographs taken before treatment (baseline) and 4 months after the last treatment" Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 14 participants completed the study |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Leheta 2011.
| Methods | A prospective, parallel, randomised controlled, hospital‐based study. Duration of the study from start until the end of follow‐up was 10 months (from October‐2008 through August‐2009) | |
| Participants | 30 participants (16 men, 14 women) with different types of atrophic acne scars were enrolled. The mean duration of acne scars was 4.8 years (range 2 – 10 years) Exclusion: "Systemic retinoids or immunosuppressive drug intake during the previous 6 months, coagulation defects or blood diseases, evidence or history of keloid scars, pregnancy or lactation, and unrealistic expectations" |
|
| Interventions | Intervention (n = 15): Percutaneous collagen induction, plus Dermaroller Comparator (n = 15): full‐concentration (100%) TCA, CROSS technique |
|
| Outcomes | Improvement in acne scars (investigator and participant): (week 4 post‐treatment) using a quartile grading scale (0 = slight improvement, < 25%; 1 = moderate improvement, 25% – 49%; 2 = significant improvement, 50% – 74%; 3 = marked improvement, > 75%) Side effects (week 4 post‐treatment) Timing: baseline, week 4, week 8, week 12, week 16 |
|
| Funding source | No funding source | |
| Declaration of interest | Nothing to be declared | |
| Notes | Egypt The Dermatology Research Ethical Committee, Faculty of Medicine, Cairo University approved the study All participants provided written informed consent |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated random sequence prepared by a statistician" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequentially numbered, sealed, opaque envelopes, and kept by a nurse not involved in the study" Comment: Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "group 1 underwent four sessions ......of PCI, and group 2 underwent four sessions ......of 100% TCA CROSS" Comment: Probably not done. Given that the control arm was easily distinguished from the treatment arm during treatment, participants and the treating dermatologist were not blinded |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "The assessor was blinded to the intervention used." Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | High risk | Quote: "group 1 underwent four sessions ......of PCI, and group 2 underwent four sessions ......of 100% TCA CROSS" Comment: No blinding of participants and the outcome assessment is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the thirty participants enrolled, twenty‐seven of completed the course of treatment. Three participants in the TCA‐100% CROSS technique group received only 1 session. Only twelve participants were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | The study protocol was obtained by contacting the investigators. The published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Leheta 2014a.
| Methods | A parallel, randomised, controlled, single‐blinded, hospital‐based study. Duration of the study from start until the end of follow‐up 13 months (from May‐2009 through June‐2010) | |
| Participants | 24 enrolled participants and 20 completed the study (12 women, 8 men, aged from 22 ‐ 40 years) with post‐acne atrophic scars Exclusion: "pregnancy or lactation, history of hypertrophic scarring or keloid formation, history of active or recurrent herpes simplex, presence of infected skin lesions, diabetes, neuromuscular disease, bleeding disorder, collagen vascular disease, acute or chronic corticosteroid or anticoagulant treatment, presence of skin cancers, warts, solar keratoses, refusal to give signed informed consent, and use of isotretinoin within 6 months before treatment" |
|
| Interventions | Intervention (n = 12): 1 session of deep peeling using a non‐hydro‐alcoholic solution of oil phenol in 60% concentration formula with few drops of croton oil/litre (lip and eyelid formula, Skintech Inc., Spain) Comparator (n = 12): 4 sessions (6 weeks apart) of percutaneous collagen induction, using the Dermaroller (model MF8; Horst Liebl CEO, Fresenheim, France), combined with TCA 20% in the same session |
|
| Outcomes | Improvement in acne scars (investigator and participant) (8 months), using a quartile grading scale (0 = minimal improvement < 25%; 1 = mild improvement 25% – 50%; 2 = moderate improvement 51% – 75%; 3 = significant improvement > 75% improvement) Side effects (investigator) (8 months) Timing: at baseline, week 6, week 12, week 18, week 32 |
|
| Funding source | No funding source | |
| Declaration of interest | Nothing to be declared | |
| Notes | Egypt Study approved by the Dermatology Research Ethical Committee, Faculty of Medicine, Alexandria University Written and signed informed consent was obtained from all participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated random sequence prepared by a statistician" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequentially numbered, sealed, opaque envelopes, and kept by a nurse not involved in the study" Comment: Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "group 1 was subjected to one session of deep peeling using phenol, and group 2 was subjected to four sessions of PCI combined with TCA 20%." Comment: Probably not done. Given that the control arm was easily distinguished from the treatment arm during treatment, participants and the treating dermatologist were not blinded |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "The assessor was blinded to the intervention used." Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | High risk | Quote: "group 1 was subjected to one session of deep peeling using phenol, and group 2 was subjected to four sessions of PCI combined with TCA 20%." Comment: No blinding of participants and the outcome assessment is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24 participants were enrolled. 4 (2 in each group) did not receive the intended treatment. Only 20 participants were analysed |
| Selective reporting (reporting bias) | Low risk | The study protocol was obtained by contacting the investigators. The published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Leheta 2014b.
| Methods | A randomised multiple‐arm single‐blinded, hospital‐based study. Duration of the study from start until the end of follow‐up 17 months (from July‐2009 through December‐2010) | |
| Participants | 39 participants with Skin Phototype III and IV with atrophic acne scars were enrolled Exclusion: "pregnancy, lactation, diabetes, history of keloids or hypertrophic scars, active infection, cancers, receiving treatment for more than 6 months" |
|
| Interventions | Trial arm 1 (n = 13): 6 sessions (4 weeks apart) of percutaneous collagen induction with 20% TCA Trial arm 2 (n = 13): 6 sessions (4 weeks apart) of 1540 nm fractional photothermolysis laser system Trial arm 3 (n = 13): 6 alternating sessions (4 weeks apart) of PCI with 20% TCA (3 sessions) and fractional photothermolysis (3 sessions) |
|
| Outcomes | Improvement of acne scar (investigator and participant) (12 months), using a quartile grading scale (0 = minimal improvement < 25%; 1 = mild improvement 25% – 50%; 2 = moderate improvement 51% – 75%; 3 = significant improvement > 75% improvement) Side effects (12 months) Timing: at baseline, and at week 48 |
|
| Funding source | No funding source | |
| Declaration of interest | Nothing to be declared | |
| Notes | Egypt | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated random sequence prepared by a statistician" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequentially numbered, sealed, opaque envelopes, and kept by a nurse not involved in the study" Comment: Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients were randomly divided into three equal groups; group 1: received six sessions …. of PCI …Group 2: received six sessions of 1540 nm fractional photothermolysis ….laser …. Group 3: received combined alternating sessions of the previously mentioned two modalities …" Comment: Probably not done. Given that the control arm was easily distinguished from the treatment arm during treatment, participants and the treating dermatologist were not blinded |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "The assessor was blinded to the intervention used." Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | High risk | Quote: "Patients were randomly divided into three equal groups; group 1: received six sessions …. of PCI …Group 2: received six sessions of 1540 nm fractional photothermolysis ….laser …. Group 3: received combined alternating sessions of the previously mentioned two modalities …" Comment: No blinding of participants and the outcome assessment is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of participants enrolled: 39. Number of participants with missing data or lost during follow‐up: 1 |
| Selective reporting (reporting bias) | Low risk | The study protocol was obtained by contacting the investigators. The published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Linkner 2014.
| Methods | A split‐face, single‐centre, randomised double‐blinded study | |
| Participants | 5 healthy adult women (age range 36 ‐ 46 years) with either ice pick, rolling, or boxcar atrophic moderate‐to‐severe acne scars on the face Exclusion: "Females who were pregnant, breastfeeding, or attempting to conceive were excluded as well as those with a history of known or suspected intolerance to any of the excipients of ALA or any of its vehicle components. Subjects with a history of cutaneous photosensitization (including porphyria or systemic lupus erythematosus), active skin malignancy or infection, and those taking any photo sensitizing medications. All medications, topical and oral, known to alter the course of acne scarring or acne vulgaris taken within two weeks of initiation or during the study period were prohibited." |
|
| Interventions | Intervention: "A solution of 20% δ‐aminolevulinic acid (commercially available as Levulan Kerastick, Dusa: ALA‐PDT) was applied topically to either the right or left sides of the face for a 60‐minute incubation period after microdermabrasion. After incubation, lesions were illuminated with 417nm blue light (Blu‐U Blue Light Photodynamic Therapy Illuminator) with irradiance of 10mW/cm2 for 1,000 seconds, with a total light dose of 10J/cm2. A therapeutic course of five consecutive treatments four weeks apart was used." Comparator: "A vehicle solution alone (vehicle‐PDT, supplied by Dusa) was applied topically to the other side of the face for a 60‐minute incubation period after microdermabrasion. After incubation, lesions were illuminated with 417nm blue light (Blu‐U Blue Light Photodynamic Therapy Illuminator) with irradiance of 10mW/cm2 for 1,000 seconds, with a total light dose of 10J/cm2. A therapeutic course of five consecutive treatments four weeks apart was used." |
|
| Outcomes | Improvement of acne scars (4 months), using the Physician’s Global Assessment of Acne Scarring scale. Timing: at baseline, week 4, week 8, week 12, week 16 Participant satisfaction (4 months) using patient questionnaire Side effects (5 months) using a 10‐point scale (0 = none; 1 – 3 = mild; 4 – 6 = moderate; 7 – 9 = severe). Timing: during and immediately after each treatment | |
| Funding source | No funding source | |
| Declaration of interest | Nothing to be declared | |
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects with moderate‐to‐severe acne scarring who were randomly assigned" Comment: Insufficient data |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment process to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A double‐blinded study" Comment: Probably done |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "Two blinded assessors reviewed pretreatment and end‐of‐study split‐ and full‐face photographs taken at each visit and evaluated acne scar severity" Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | Low risk | Quote: "A double‐blinded study" Comment: Probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 participants were enrolled with 5 completing the study |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Manuskiatti 2013.
| Methods | A split‐face prospective, randomised, comparative interventional trial | |
| Participants | 24 Thai adults aged 22 – 51 (mean 29.5, both genders) with skin phototype IV. "All participants had shallow or deep boxcar scars or both on their faces for least 6 months before entering the study. 20 participants completed the study" Exclusion: "People who were pregnant or lactating, had concomitant treatment to involved skin areas, had a propensity for keloid scarring, had received isotretinoin, or had undergone filler injections or ablative or non‐ablative laser skin resurfacing procedures within the preceding 12 months" |
|
| Interventions | Intervention: 1 side of the face treated with 1 pass of ablative fractional Er:YAG laser with a pulse duration of 350 ls and an energy of 14 mJ with average of 5% skin surface coverage. Participants received 2 treatment sessions with a 2‐month interval. Comparator: The other side of the face treated with 1 pass of ablative fractional CO₂ laser with a pulse duration of 950 ls and a mean energy of 13.75 (12.5 – 15) mJ with average of 5% skin surface coverage. Participants received 2 treatment sessions with a 2‐month interval |
|
| Outcomes | Improvement of acne scars by physician: 6 months using a quartile grading scale (0 = < 25%; 1 = 25% – 50%; 2 = 51% – 75%; 3 = > 75% improvement)
Improvement of acne scars by participants: 6 months as follows: slightly better (< 25%), fair (25% – 50%), good
(51% – 75%), and excellent (≥ 76%)
Side effects (6 months), using a 10‐point pain scale (0 = no pain to 10 = severe pain)
Objective assessment of scar volume (6 months) using an ultraviolet A (UVA) light video camera (Visioscan VC 98; Courage‐Khazaka, Köln, Germany) with analysis software. Timing: at baseline and 3 and 6 months after the final treatment Timing: at baseline, at week 8, and 1, 3, and 6 months after the final treatment session |
|
| Funding source | No funding source | |
| Declaration of interest | Nothing to be declared | |
| Notes | Thailand Informed consent was obtained from all study subjects Institutional Review Board approved the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a random digit table" Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment process to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "Two blinded medical assessors independently assessed clinical improvement in the appearance of acne scars." Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20/24 participants enrolled completed the treatment protocol and were followed through the end of the study. 4 participants were withdrawn from the study because 3 had scheduling conflicts and the other was unable to be contacted during follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Min 2009.
| Methods | A single‐blinded randomised comparative split‐face study | |
| Participants | 19 participants (7 men and 12 women) with atrophic acne scarring. Ages ranged from 21 to 30 (average 23), and Fitzpatrick skin types were IV or V. Exclusion: "known photosensitivity, pregnancy or lactation, a previous history of hypertrophic or keloidal scarring, the use of isotretinoin, and a previous history of facial laser treatment or of a surgical procedure within 6 months of study enrolment. Patients with a medical condition that might have influenced the wound healing process" |
|
| Interventions | Intervention: 1 facial side was treated using non‐overlapping pulses of a long‐pulse Nd:YAG laser at a fluence of 50 to 70 J/cm² and a 50‐ to 100‐ms pulse duration using a 7‐mm spot size. 2 passes of laser treatment were delivered at each session. All participants received 4 treatment sessions at 2‐week intervals. Comparator: at the same session, contralateral side was treated using a combined 585/1064‐nm laser (Cynergy, Cynosure Inc., Westford, MA). 2 passes of laser treatment were delivered at each session. All participants received 4 treatment sessions at 2‐week intervals |
|
| Outcomes | Improvement of acne scars (14 weeks), as % improvements (0% – 100%) from baseline using ECCA scores
Participant satisfaction (14 weeks), using a numerical scale ranging from 0 (neutral) to 10 (highly satisfied)
Side effects (14 weeks)
Histological analyses: 8 weeks before treatment began and again at 8 weeks after final treatment Timing: at baseline, week 2, week 4, week 6, week 10, week 14 |
|
| Funding source | No funding source | |
| Declaration of interest | Nothing to be declared | |
| Notes | Korea Study approved by the Institutional Review Board of Seoul National University Hospital Written informed consent was obtained from all participants before enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly selected facial sides" Comment: Insufficient data |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment process to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "A single‐blinded" Comment: Probably not done |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "Two blinded dermatologists assessed acne scar improvements" Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | High risk | Quote: "A single‐blinded" Comment: No blinding of participants and the outcome assessment is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The 19 participants completed the 14‐week study |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Munavalli 2013.
| Methods | A split‐face randomised multicentre, double‐blind, placebo‐controlled trial | |
| Participants | Healthy adults (62 women and 47 men, aged 19 ‐ 65 years) with moderate or severe facial acne scarring on both cheeks Exclusion: "Hypertrophic acne scarring or numerous icepick acne scars in the treatment area, aesthetic procedures (e.g. fractional or traditional ablative/non‐ablative laser resurfacing, subcision, microdermabrasion, chemical peels) to the treated area within the past 12 months, or ever previously received injectable fillers in the treated area. History of heavy smoking, alcohol or drug abuse, or steroid treatment" |
|
| Interventions | Intervention: 1 cheek of each participant received autologous fibroblasts (LaViv, azficel‐T, Fibrocell Sciences, Inc, Exton, PA) (10 – 20 million cells/mL) injected into the high papillary dermis at a maximum dose of 2 mL per treatment for 3 treatments about 14 days apart Comparator: The other cheek received vehicle control (dye‐free, protein‐free cell culture medium) injected into the high papillary dermis at a maximum dose of 2 mL per treatment for 3 treatments about 14 days apart |
|
| Outcomes | Improvement of acne scars (participant and evaluator): 4 months using a 5‐point scale (–2 = much worse; –1 = worse; 0 = no change; +1 = improved; +2 = much improved) by evaluators and a 5‐point evaluator live acne scar assessment scale (0 = clear; 1 = very mild; 2 = mild; 3 = moderate; 4 = severe) by participants
Side effects (4 months) Timing: 1, 2, 3, and 4 months after the third treatment |
|
| Funding source | This study was funded by Fibrocell Science, Inc | |
| Declaration of interest | Drs. Munavalli, Smith, and Weiss are consultants and serve on the advisory board for Fibrocell Science, Inc. Mr. Maslowski is an employee of Fibrocell Sciences, Inc | |
| Notes | USA A centralised Institutional Review Board, Chesapeake Research Review, Inc., reviewed and approved the protocol Informed consent forms and written informed consent was obtained from all participants at 7 USA sites before study participation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The cheeks of each subject were randomised" Comment: Insufficient data |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment process to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A double‐blind, placebo‐controlled trial." Comment: Probably done |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "Evaluators were blinded to the treatment each cheek had received." Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | Low risk | Quote: "A double‐blind, placebo‐controlled trial." Comment: Probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 96/99 participants completed the series of 3 treatments. Of the 99 treated, there were 7 early study exclusions. 1 participant withdrew consent for reasons unrelated to adverse events, and 6 were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes that are of interest in the review. |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Rongsaard 2014.
| Methods | A randomised split‐faced clinical study | |
| Participants | 20 Thai adults (12 men, 8 women) aged 18 – 55 with Fitzpatrick skin types III ‐V and atrophic acne scars on both cheeks Exclusion: "pregnancy, lactation, photosensitivity, electrical implantation, immunocompromise, history of deep chemical peeling or laser resurfacing, botulinum toxin or filler injection in the previous 6 months, history of hypertrophic scars and keloids, use of isotretinoin within 6 months, allergy to anaesthesia, active inflammatory skin disease or pre‐malignant and malignant lesions in the treatment area, and history of herpes simplex or herpes zoster on the face" |
|
| Interventions | Intervention: 1 side of the face treated using the fractional bipolar radiofrequency (RF) device (eMatrix, Syneron, Haifa, Israel) with 64‐electrode‐pin disposable tips was Program C (53 – 59 mJ/pin for 2 passes). 3 treatment sessions were done at 4‐week intervals Comparator: The other side of the face treated with the fractional erbium‐doped glass 1550‐nm device (Fraxel re:store DUAL1550/1927, Solta Medical, Hayward, CA) with energy settings ranged from 30 ‐ 50 mJ/MTZ, with treatment levels 4 – 5 for 8 passes. 3 treatment sessions were done at 4‐week intervals |
|
| Outcomes | Improvement of acne scars (1 month), using a grading scale as follows: 0 = no improvement; 1 = < 25% (mild) improvement; 2 = 25% – 50% (moderate) improvement; 3 = 51% – 75% (good) improvement; 4 = > 75% (excellent) improvement Improvement in facial texture (1 month) by comparing the texture scores obtained from the Complexion Analysis System before and after treatment Participant satisfaction (1 month) using a grading scale (0 = dissatisfied; 1 = less satisfied; 2 = moderately satisfied; 3 = very satisfied; 4 = most satisfied) Side effects (1 month) Timing: 1 month |
|
| Funding source | No available data | |
| Declaration of interest | Nothing to be declared | |
| Notes | Thailand Participants signed an informed consent form for participation in the study The Mae Fah Luang Ethical Committee, Chiang Rai, Thailand, approved the study protocol |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The researcher generated randomisation sequence using random allocation software." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Concealing the sequence in opaque envelopes" Comment: Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: ".....one side with fractional bipolar RF and the other with fractional erbium‐doped glass" Comment: Given that the control arm was easily distinguished from the treatment arm during treatment, the treating dermatologist were not blinded. There is insufficient data for blinding of the participants |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "Three masked dermatologists evaluated clinical improvement of acne scars. They independently evaluated improvement in acne scars by comparing the photographs taken before and after three treatment sessions." Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19/20 participants completed 3 treatment sessions. 1 man with Fitzpatrick skin type III withdrew from the study because he developed side effects in the form of prolonged dyspigmentation, which became evident after the second treatment session and negatively affected his professional life. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Sage 2011.
| Methods | A prospective, randomised, split‐face, single‐blind study | |
| Participants | 10 participants aged 18+ and had approximately symmetric depressed and rolling types of acne scars. Participants were 6 white, one Middle‐Eastern, one Hispanic, one Asian, and one African‐American (mean age 50, range 33 – 65, skin types II – V) Exclusion: "Active or unstable acne, ice‐pick or boxcar type scarring, history of isotretinoin therapy within the last 6 months, and history of prior resurfacing or cosmetic procedure within the last 6 months" |
|
| Interventions | Intervention: 1 half of the face treated with subcision using an 18‐gauge Nokor subcision needle (Becton Dickinson & Co, Franklin Lakes, NJ) for a single session Comparator: The other half was injected with the NSPC filler using the supplied 0.5 mL 27‐gauge prepackaged syringe to the base of the depressed scars for a single session |
|
| Outcomes | Overall cosmetic outcome (investigator and participant) (6 months), using a scale from 1 to 5 (1 = worse than before treatment; 2 = no change; 3 = minimal disappearance; 4 = moderate disappearance; 5 = complete disappearance)
Side effects (investigator and participant) (1 week) on a tolerability scale from 0 to 3 (0 = no symptoms; 1 = mild symptoms; 2 = moderate symptoms; 3 = severe symptoms)
Side effects (investigator and participant) (6 months) Timing: 3 and 6 months after treatment |
|
| Funding source | The Cosmetic Surgery Foundation for Education, Research, and Patient Safety, Inc | |
| Declaration of interest | Nothing to be declared | |
| Notes | USA Study approved by the Henry Ford Health System Institutional Review Board and Ethics Committee Informed consent was obtained from all participants before enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A nurse flipped a coin to determine randomisation." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation could not be foreseen due to use of coin tossing for randomisation." Comment: Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "single‐blind study" "For the subcision‐treated side, an 18‐gauge Nokor subcision needle .....was used.... The contralateral side of the face was injected with the NSPC using the supplied ... syringe." Comment: Probably not done. Given that the control arm was easily distinguished from the treatment arm during treatment, participants and the treating dermatologist were not blinded |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "Two blinded physicians evaluated results according to clinical observations of the study subjects and review of clinical photographs." Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | High risk | Quote: "single‐blind study" "For the subcision‐treated side, an 18‐gauge Nokor subcision needle .....was used.... The contralateral side of the face was injected with the NSPC using the supplied ... syringe." Comment: No blinding of participants and the outcome assessment is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 10 participants completed the 1‐week post‐procedure follow‐up visit. 9/10 completed the 3‐month follow‐up visit. All 10 completed the 6‐month follow‐up visit |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Tanzi 2004.
| Methods | A split‐face prospective clinical and histologic study | |
| Participants | 20 consecutive patients with mild to moderate atrophic facial scars (mean age of 36.7 years; skin phototype V) Exclusion: "a history of isotretinoin use, dermabrasion, phenol peel, or temporary filler (e.g. collagen, fat) injections within 3 years. Any prior history of injectable silicone or other permanent fillers in the facial areas" |
|
| Interventions | Intervention: 1 facial half received treatment with a 1320‐nm Nd:YAG laser (CoolTouch; CoolTouch Corp., Auburn, CA). The 1320‐nm Nd:YAG laser applied fluences ranging 12 to 17 J/cm² (average of 14.8 J/cm²) through a 10‐mm spot size for 2 passes over the treatment area. Each participant received 3 laser treatments by a single operator (ELT) using an identical laser technique at 4‐week intervals. Comparator: The other half received treatment with a 1450‐nm midinfrared diode (SmoothBeam; Candela Corp., Wayland, MA). The 1450‐nm diode laser was used at fluences ranging 9 to 14 J/cm² through a 6‐mm spot size in a single non‐overlapping pass. Each participant received 3 laser treatments by a single operator (ELT) using an identical laser technique at 4‐week intervals |
|
| Outcomes | Participant satisfaction (6 months) on a scale of 1 (lowest) to 10 (highest) at the end of the study
Improvement in the quality of skin texture (6 months) using a quartile grading scale (1 = < 25%, minimal to no improvement; 2 = 25% to 50%, moderate improvement; 3 = 51% to 75%,marked improvement; 4 = > 75%,near total improvement). Timing: at baseline and at 1, 3, 6, and 12 months after the final laser treatment
Improvement in roughness, average values (6 months) using a 13 18‐mm in vivo 3‐dimensional microtopography
skin imaging system. Timing: at baseline and at 1, 3, 6, and 12 months after the final laser treatment
Side effects (6 months)
Histological evaluation: at baseline, immediately after the first laser treatment, and at 1, 3, 6, and 12 months after the final laser treatment Timing: at baseline, week 4, week 8, and at 1, 3, 6, and 12 months after the final laser treatment |
|
| Funding source | This was supported by the ASDS Cutting Edge research grant programme | |
| Declaration of interest | Nothing to be declared | |
| Notes | USA The study was done after Institutional Review Board–approved informed consent was obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Facial halves were randomly assigned" Comment: Insufficient data |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment process to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "randomly received three successive monthly treatments with a long‐pulsed 1320‐
nm Nd:YAG laser on one facial half and a long‐pulsed 1450‐nm diode laser on the contralateral facial half." Comment: Given that the control arm was easily distinguished from the treatment arm during treatment, the treating dermatologist were not blinded. There is insufficient data for blinding of the participants |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | The clinical response was evaluated by 2 dermatologists independent of the investigator (blinded assessments). Evaluations were based on digital photography in which the follow‐up photographs were randomly presented for comparison with the known baseline photograph |
| Blinding of outcome assessment (detection bias) Participant‐reported | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed treatment sessions and all were analysed |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Zhang 2013.
| Methods | An evaluator‐blinded, randomised, comparative split‐face study | |
| Participants | 33 Chinese adults with mild to severe atrophic acne scars on both sides of the face, 14 women and 19 men, aged 19 ‐ 34 (average 26.4 ± 3.7) with Fitzpatrick skin types III and IV Exclusion: "Pregnancy; breastfeeding; history of keloid tendency; immunosuppression; photosensitivity or current use of photosensitive medication; oral isotretinoin use in the preceding 6 months; use of topical retinoids in the preceding 2 weeks; active dermatitis; infection or malignancy over the treatment area; and having received light source, radiofrequency, or laser skin resurfacing treatments in the 6 months before the study". |
|
| Interventions | Intervention: 1 facial half received treatment with a CO₂ fractional laser system (FS) (10600‐nm Ultrapulse Encore; Lumenis Inc., Santa Clara, CA) with 20 to 25 mJ, density, 2 to 4 (10% – 20% coverage/cm² per pass), 300 Hz, using the Deep FX mode and 1 pass without overlapping. All participants received 3 treatment sessions at intervals of 6 to 12 (average 8) weeks Comparator: The other half received treatment with a fractional micro‐plasma radiofrequency (RF) device (Accent; Alma Lasers, Caesarea, Israel). 4 passes of the roller tip at 50 ro 60 W. All participants received 3 treatment sessions at intervals of 6 to 12 (average 8) weeks |
|
| Outcomes | Overall acne scar improvement (6 months) using ECCA scores Individual acne scar type improvement (6 months) Participant satisfaction (6 months), as follows: very satisfied, satisfied, slightly satisfied, or unsatisfied Side effects (6 months), pain was evaluated using 10‐cm VAS, with 0 being no pain and 10 being extremely painful. Immediately after each treatment Histologic analysis. Timing: immediately after first treatment | |
| Funding source | No available data | |
| Declaration of interest | Nothing to be declared | |
| Notes | China Study approved by Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China. Informed consent was obtained from all participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Facial halves were randomly to assigned." Comment: Insufficient data |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment process to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "received three sessions of a randomized split‐face treatment of fractional microplasma RF or CO₂ FS" Given that the control arm was easily distinguished from the treatment arm during treatment, the treating dermatologist were not blinded. There is insufficient information for blinding of participants. |
| Blinding of outcome assessment (detection bias) Investigator‐ assessed | Low risk | Quote: "Two unbiased, board‐certified dermatologists conducted blinded clinical assessments of the treatment areas using comparative photographs." Comment: Probably done |
| Blinding of outcome assessment (detection bias) Participant‐reported | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 33 participants completed 3 treatments and a 6‐month post‐procedure follow‐up visit |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes that are of interest in the review |
| Other bias | Low risk | The study appears to be free of other sources of bias |
ALA: aminolevulinic acid CROSS: chemical reconstruction of skin scars ECCA: échelle d’évaluation clinique des cicatrices d’acné NSPC: natural source porcine collagen PCI: percutaneous collagen induction PDT: photodynamic therapy PMMA: polymethylmethacrylate PRP: platelet‐rich plasma TCA: trichloroacetic acid VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmed 2014 | The study was a non‐randomised comparative clinical trial. There is notable inconsistency in methods between the published manuscript and the thesis manuscript. Investigators did not respond to clarify these inconsistencies |
| Alexis 2011 | The study assessed the efficacy and safety of 1 intervention (a 1550 nm erbium‐doped fractionated laser with 40mJ and treatment level 4 (11% surface area coverage) versus 40mJ and treatment level 7 (20% surface area coverage)) |
| Alster 1996 | The study was not a RCT |
| Azzam 2013 | The study was not a RCT |
| Balighi 2008 | The study was not a RCT |
| Bjørn 2014 | The study assessed the efficacy and safety of 1 intervention (fractional CO₂ laser at 1‐month versus 3‐month intervals) |
| Dreno 2007a | The study included different type of participants |
| Gadkari 2014 | The study was a non‐randomised comparative clinical trial |
| Gawdat 2014 | The study was not a RCT |
| Goldman 1999 | The study recruited individuals with photo‐damaged skin |
| Jung 2010 | The study assessed the efficacy and safety of 1 intervention (lower‐fluence, higher‐density versus higher‐fluence, lower‐density treatment with a CO₂ fractional Laser) |
| Kim 2009a | The trial did not allocate different interventions at random within participants |
| Laubach 2008 | The study assessed the effect of penetration depth of 1 intervention (non‐ablative fractional laser) |
| Laubach 2009 | The study assessed the efficacy and safety of 1 intervention (non‐ablative fractional laser using 6 mJ/MTZ versus 70 mJ/MTZ) |
| Lee 2014 | The study was not a RCT |
| Mohammed 2013 | The study was a non‐randomised comparative clinical trial. There is notable inconsistency in methods between the published manuscript and the thesis manuscript. Investigators did not respond to clarify these inconsistencies |
| Nofal 2014 | The study was a non‐randomised comparative clinical trial. There is notable inconsistency in methods between the published manuscript and the thesis manuscript. Investigators did not respond to clarify these inconsistencies |
| Sharad 2011 | The study was not a RCT |
| Srivastava 2009 | The study assessed the efficacy and safety of 1 intervention (1550‐nm erbium fractionated laser 10 mJ versus 40 mJ) |
| Tanghetti 2013 | The study assessed the efficacy of 1 intervention (deep versus superficial non‐ablative fractional laser) |
| Vejjabhinanta 2014 | The study was not a RCT |
| Yaghmai 2005 | The study assessed the efficacy and safety of 1 intervention (2 different wavelengths (1064nm versus 1320 nm) of the same non‐ablative Nd:Yag laser) |
| Yuan 2014 | The study assessed the efficacy of 1 intervention (the same fractional CO₂ laser using different fluences and densities) |
CO₂: carbon dioxide Nd:Yag: neodymium‐doped yttrium aluminium garnet RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Dadzie 2006.
| Methods | This was a double‐blind RCT |
| Participants | 20 patients with facial atrophic acne scarring (age range 19 – 53 years) |
| Interventions | Isolagen treatment (3 injection sessions at 2‐week intervals) in either the right or left cheek, with placebo saline injection used on the nontreated side |
| Outcomes | Improvement in roughness and peak height using facial profilometry Improvement,using a 5‐point scale, by participant and investigator of acne scarring at 6 months post‐Isolagen injection |
| Notes | Abstract only. Abstract does not contain enough information for 'Risk of bias' assessment and completion of the Characteristics of included studies table |
Dreno 2003.
| Methods | Double‐blind randomised study |
| Participants | Patients with acne scarring and post‐inflammatory pigmentation |
| Interventions | Diacneal® cream (combining retinaldehyde 0.1% and glycolic acid 6%) compared with excipient |
| Outcomes | Evolution of the scarring score (a scale for scarring with 5 gradings: pigmentation, atrophic scars (with 3 stages: V, U, W), excoriations, elastolysis and hypertrophic scaring) at D28, D56 and D78 and the global opinion of the participants Tolerance (erythema, dryness, pruritis, burning, stinging), new scarring and counts of retentional and inflammatory lesions, cosmetic qualities of the products |
| Notes | Abstract only. Abstract does not contain enough information for 'Risk of bias' assessment and completion of the Characteristics of included studies table |
Ghaly 2012.
| Methods | RCT |
| Participants | 30 patients (skin phenotypes III to V, mean age 32.7 years) with moderate to severe facial post‐acne scarring were randomly divided into 2 equal groups |
| Interventions | Group A received 2 ‐ 3 sessions with 2940‐nm ablative erbium: YAG laser at 4‐ to 8‐week intervals Group B underwent 6 ‐ 9 treatments with 1540‐nm non‐ablative fractional erbium laser at 4‐ to 6‐week intervals |
| Outcomes | The overall average clinical improvement Side effects (at 1, 2, and 3 months after the last session) |
| Notes | Abstract only. Abstract does not contain enough information for 'Risk of bias' assessment and completion of the Characteristics of included studies table |
Grimes 2014.
| Methods | A prospective, randomised, double‐blind, controlled, multicentre cross‐over study |
| Participants | Participants were required to have at least 4 acne scars in the facial area that met the following criteria: soft‐contoured, rolling scars that were distensible and moderate to severe (3 or 4) on a validated 4‐point (1 ‐ 4) acne scar rating scale (ASRS) |
| Interventions | Participants were randomised to receive either PMMA‐collagen or control injections of saline. At month 6, those who had received control injections could be treated with PMMA‐collagen |
| Outcomes | Participants were deemed responders if 50% or more of their scars improved by at least 2 grades on the ASRS by both investigators and participants self assessments Visits were scheduled every 2 weeks for the first month, then at month 3 and 6. All participants were followed for an additional 6 months after cross‐over |
| Notes | Abstract only. Abstract does not contain enough information for 'Risk of bias' assessment and completion of the Characteristics of included studies table |
Munavalli 2011.
| Methods | A pilot RCT |
| Participants | 12 patients (Fitzpatrick skin types II–V) with moderate to severe acne scarring |
| Interventions | A vacuum‐assisted radiofrequency (RF) device was randomised to one side, with contralateral side as control |
| Outcomes | Unlabeled baseline and 6‐month photography were evaluated by a blinded, non‐treating physician assessor using a quartile grading scale to denote improvement |
| Notes | Abstract only. Abstract does not contain enough information for 'Risk of bias' assessment and completion of the Characteristics of included studies table |
Qian 2014.
| Methods | A RCT |
| Participants | 27 patients with indented acne scars, aged 18 ‐ 40 years(skin type Ⅱ ‐ Ⅴ) |
| Interventions | 14 participants received CO₂ fractional laser on a random cheek, and ER‐YAG fractional laser on the other cheek. In control group, 13 participants received CO₂ fractional laser on a random cheek, and the other cheek left untreated |
| Outcomes | ECCA grading scale (by photographs) Recovery and adverse effects (immediately after the treatment, at 7 postoperative days, 1 month, 3 months and 6 months) |
| Notes | Abstract only. Abstract does not contain enough information for 'Risk of bias' assessment and completion of the Characteristics of included studies table |
Sachsenberg‐Studer 2002.
| Methods | A single‐centre, randomised, comparative, double‐blind, intra‐individual (left/right) study |
| Participants | 14 patients were recruited undergoing laser skin resurfacing for bilateral acne scars |
| Interventions | Pre‐treatment: retinaldehyde (RAL) and retinoic acid (RA) at 0.05% on randomly‐assigned hemiface for 2 weeks Skin resurfacing: Er: YAG laser (wavelength 2940nm, pulse length 350ms, energy 5J/cm2). Post‐treatment 1 week after laser therapy, continued for 5 weeks: RAL and RA on the same hemifacial sites as before |
| Outcomes | Chromametry assessment for facial erythema Histological evaluation |
| Notes | Abstract only. Abstract does not contain enough information for 'Risk of bias' assessment and completion of the Characteristics of included studies table |
Sarkate 2015.
| Methods | A split‐face placebo‐controlled study |
| Participants | 30 patients with different Goodman and Baron (GB) qualitative acne grades |
| Interventions | Each participant received BT intradermally in a dose of 10 – 20 units, depending on the grade of scarring on 1 half of face at baseline and at 4 weeks. The other half of the face was injected with an equal amount of normal saline (NS) |
| Outcomes | Final assessment was at 8 weeks by ECCA score, GB grade, assessment of clinical photographs by 2 independent observers at baseline and after 8 weeks on a 4‐point scale, and surface profilometry of facial imprints taken on alginate moulds at baseline and after 8 weeks |
| Notes | Abstract only. Abstract does not contain enough information for 'Risk of bias' assessment and completion of the Characteristics of included studies table |
Sarnoff 2012.
| Methods | A split‐face pilot RCT |
| Participants | 10 patients, Fitzpatrick Skin Types I ‐ V, 3 men, 7 women, aged 26 – 66, with atrophic facial acne scars |
| Interventions | Participants were randomised to receive a single treatment using a combination of non‐ablative fractional 1440nm Nd:YAG laser resurfacing and ablative fractional CO₂ laser resurfacing on 1 side of the face and ablative fractional CO₂ laser resurfacing alone on the contralateral side |
| Outcomes | Physician evaluation of blinded pre‐operative and postoperative photos was performed at 3 and 6 months postoperatively. Participants evaluated their results at 3 and 6 months post‐operatively |
| Notes | Abstract only. Abstract does not contain enough information for 'Risk of bias' assessment and completion of the Characteristics of included studies table |
ECCA: échelle d’évaluation clinique des cicatrices d’acné Nd:YAG: neodymium‐doped yttrium aluminium garnet PMMA: polymethylmethacrylate RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT02216864.
| Trial name or title | Pilot split‐face randomised, evaluator‐blinded study on the effect of multiple subcisions on rolling acne scars |
| Methods | A randomised, split‐face, single‐blind (outcomes assessor) trial Primary purpose: treatment |
| Participants | 20 individuals with rolling acne scars Ages eligible for study: 18 ‐ 65 years Genders eligible for study: both |
| Interventions | Experimental: Multiple subcision No intervention: control (on the other side of the face) |
| Outcomes | Change in acne scarring compared to baseline after treatments: time frame: baseline and 36 weeks The change in acne scarring is measured using a quantitative global scarring grading system to compare baseline to the treatment |
| Starting date | August 2014 |
| Contact information | Contact: Emily Poon, PhD 312‐695‐4761 research.nuderm@northwestern.edu Principal Investigator: Murad Alam, MD |
| Notes |
NCT02216864 Sponsor: Northwestern University Country: USA |
NCT02643628.
| Trial name or title | A pilot study to evaluate the safety and effectiveness of microneedling and bellafill to treat facial acne scars |
| Methods | Open‐label, parallel, randomised, multicentre, prospective trial Primary purpose: treatment |
| Participants | 45 individuals with distensible atrophic acne scars Ages eligible for study: 21+ Genders eligible for study: both |
| Interventions | Arm 1: microneedling Arm 2: microneedling followed by injectable filler |
| Outcomes | Acne scar assessment scale: time frame 6 months |
| Starting date | November 2015 |
| Contact information | Nancy Seretta (nserreta@sunevamedical.com) |
| Notes |
NCT02643628 Sponsor: Suneva Medical, Inc Country: USA |
Differences between protocol and review
Main text: in the protocol, our objective was 'To assess the effects of interventions for treating facial acne scars' . We also referred to facial acne scars in the Types of studies section and the Criteria for considering studies for this review. We have revised the objectives by removing the word 'facial' to match our title (Interventions for acne scars), in order not to miss that we were also interested in including any acne scarring on the back.
Electronic searches: in the protocol, we had planned to search the metaRegister of Controlled Trials (www.controlled‐trials.com), but in the review, we searched the ISRCTN registry. The metaRegister of Controlled Trials was a section of the ISRCTN website, but the service is under review by its providers.
Data extraction and management: although not planned in the protocol, we developed a computer database tool to enable us to do the data extraction.
Unit of analysis issues: for trials using a split‐face design, we intended to perform a paired analysis using the generic inverse variance method and undertake a sensitivity analysis where imputations exist, but paired data were unavailable and we were not able to adjust for the within‐participant variability.
Dealing with missing data: in the protocol, we planned " For continuous outcomes, we will use the last observation carried forward for imputation of those with missing outcome data if individual participant data are available." However, we did not undertake these plans in the review because of insufficient data. In future updates of this review we will revisit our methodology.
Assessment of reporting biases: if there had been sufficient studies, we planned to investigate reporting biases (such as publication bias) for primary outcomes using funnel plots.
Subgroup analysis and investigation of heterogeneity: there were not enough studies to conduct the planned subgroup analysis.
Sensitivity analysis: we did not perform any sensitivity analysis due to a paucity of studies in each comparison.
Summary of Findings: We graded the quality of the evidence for six outcomes and presented them in our Summary of Findings tables as planned. We include an additional outcome, 'Serious or severe adverse events', to Table 4, based on our second primary outcome ‘Participants with adverse effects serious or severe enough to have caused their withdrawal from the study’, because we think it a critical outcome for decision making.
Contributions of authors
RAH was the contact person with the editorial base. AN co‐ordinated contributions from the co‐authors and wrote the final draft of the review. KS, SD, VH screened papers against eligibility criteria. KS and SD obtained data on ongoing and unpublished studies. KS, SD, AN, CC appraised the quality of papers. KS, SD, RAH extracted data for the review and sought additional information about papers. KS, SD, RAH entered data into Review Manager 5. AN, CC, RAH analysed and interpreted data. AN worked on the Methods sections. RAH, KS, SD, CC drafted the clinical sections of the Background and responded to the clinical comments of the referees. AN, CC, RAH responded to the methodology and statistics comments of the referees. RAH drafted the Results and the Discussion sections and responded to the referee comments. AL reviewed the protocol and final review, provided clinical expertise and responses to referee comments. HZ made an intellectual contribution to the review and responded to the clinical comments of the referees. RAH wrote the final draft of the review. RAH is the guarantor of the update. All authors approved the final version of the protocol and the review prior to publication.
Disclaimer
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Sources of support
Internal sources
Egyptian Center for Evidence Based Medicine (EC EBM), Egypt.
External sources
-
The National Institute for Health Research (NIHR), UK.
This project was supported by the National Institute for Health Research, via Cochrane Incentive Award funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health. The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Rania Abdel Hay has been involved in the following included studies for this Cochrane review. She was not involved in 'Risk of bias' assessment, or data extraction and analysis of these studies: Leheta 2011, Leheta 2014a, and Leheta 2014b. Khalid Shalaby: nothing to declare. Hesham Zaher: nothing to declare. Vanessa Hafez: nothing to declare. Ching‐Chi Chi: nothing to declare. Sandra Dimitri: nothing to declare. Ashraf Nabhan: Over the last 12 months, Novartis provided honoraria to speak at educational events (content: Designing clinical research). Alison Layton: "Over the last three years, the following companies have invited advice, supported educational events, provided unrestricted research grants, or I have acted as Clinical Investigator (CI)/Principle Investigator (PI) for their clinical trials. Galderma: 1. PI for two clinical trials; 2. CI for peer‐reviewed basic science research study provided with an unrestricted educational grant; 3. member of Global and European Acne panels sponsored by Galderma with unrestricted educational grants; 4. honoraria provided to speak at educational events (unrestricted content); and 5. honoraria provided for consultancy fees as a member of a drug‐monitoring committee. GlaxoSmithKline: consultancy fee for supporting the development of a trial protocol. MEDA: honorarium for speaking at educational symposium (content unrestricted). LeoPharma: PI in a clinical trial. Pfizer: PI in a clinical study. Novartis: PI in a clinical study. L'Oreal: consultancy fee for monitoring a clinical study. I am not affiliated to or hold shares in any one specific company. I will not be involved in the rating and data extraction of any of these studies for this Cochrane review."
Hywel Williams, Cochrane Dermatology Editor: "I work for the NIHR Health Technology Assessment (HTA), which is funding part of this work."
New
References
References to studies included in this review
Alam 2014 {published data only}
- Alam M, Han S, Pongprutthipan M, Disphanurat W, Kakar R, Nodzenski M, et al. Efficacy of a needling device for the treatment of acne scars: a randomized clinical trial. JAMA Dermatology 2014;150(8):844‐9. [PUBMED: 24919799] [DOI] [PubMed] [Google Scholar]
- Pongprutthipan M, Nodzenski M, Veledar E, West D, Dubina M, Alam M, et al. A randomized controlled trial to assess the efficacy of a needling device for the treatment of acne scars. Lasers in Surgery and Medicine 2014;46:4. [EMBASE: 71594493] [Google Scholar]
Asilian 2011 {published data only}
- Asilian A, Salimi E, Faghihi G, Dehghani F, Tajmirriahi N, Hosseini SM. Comparison of Q‐Switched 1064‐nm Nd: YAG laser and fractional CO2 laser efficacies on improvement of atrophic facial acne scar. Journal of Research in Medical Sciences 2011;16(9):1189‐95. [EMBASE: 2011598154] [PMC free article] [PubMed] [Google Scholar]
Bernstein 2001 {published data only}
- Bernstein EF, Ferreira M, Anderson D. A pilot investigation to subjectively measure treatment effect and side‐effect profile of non‐ablative skin remodeling using a 532 nm, 2 ms pulse‐duration laser. Journal of Cosmetic & Laser Therapy 2001;3(3):137‐41. [EMBASE: 2002362049] [DOI] [PubMed] [Google Scholar]
Chae 2015 {published data only}
- Chae WS, Seong JY, Jung HN, Kong SH, Kim MH, Suh HS, et al. Comparative study on efficacy and safety of 1550 nm Er: Glass fractional laser and fractional radiofrequency microneedle device for facial atrophic acne scar. Journal of Cosmetic Dermatology 2015;14(2):100‐6. [EMBASE: 2015071568] [DOI] [PubMed] [Google Scholar]
Cho 2010 {published data only}
- Cho SB, Lee SJ, Cho S, Oh SH, Chung WS, Kang JM, et al. Non‐ablative 1550‐nm erbium‐glass and ablative 10 600‐nm carbon dioxide fractional lasers for acne scars: a randomized split‐face study with blinded response evaluation. Journal of the European Academy of Dermatology and Venereology 2010;24(8):921‐5. [EMBASE: 2010378178] [DOI] [PubMed] [Google Scholar]
Erbağci 2000 {published data only}
- Erbağci Z, Akçali C. Biweekly serial glycolic acid peels vs. long‐term daily use of topical low‐strength glycolic acid in the treatment of atrophic acne scars. International Journal of Dermatology 2000;39(10):789‐94. [EMBASE: 2000387524] [DOI] [PubMed] [Google Scholar]
Faghihi 2015 {published data only}
- Faghihi G, Nouraei S, Asilian A, Keyvan S, Abtahi‐Naeini B, Rakhshanpour M, et al. Efficacy of punch elevation combined with fractional carbon dioxide laser resurfacing in facial atrophic acne scarring: a randomized split‐face clinical study. Indian Journal of Dermatology 2015;60(5):473‐8. [EMBASE: 2015376448] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedelund 2010 {published data only}
- Hedelund L, Moreau KER, Beyer DM, Nymann P, Haedersdal M. Fractional nonablative 1,540‐nm laser resurfacing of atrophic acne scars. A randomized controlled trial with blinded response evaluation. Lasers in Medical Science 2010;25(5):749‐54. [EMBASE: 2010472544] [DOI] [PubMed] [Google Scholar]
Hedelund 2012 {published data only}
- Hedelund L, Haak CS, Togsverd‐Bo K, Bogh MK, Bjerring P, Haedersdal M. Fractional CO2 laser resurfacing for atrophic acne scars: A randomized controlled trial with blinded response evaluation. Lasers in Surgery and Medicine 2012;44(6):447‐52. [EMBASE: 2012412028] [DOI] [PubMed] [Google Scholar]
Karnik 2014 {published data only}
- Karnik J, Baumann L, Bruce S, Callender V, Cohen S, Grimes P, et al. A double‐blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. Journal of the American Academy of Dermatology 2014;71(1):77‐83. [EMBASE: 2014426024] [DOI] [PubMed] [Google Scholar]
Kim 2009 {published data only}
- Kim HJ, Kim TG, Kwon YS, Park JM, Lee JH. Comparison of a 1,550 nm Erbium: glass fractional laser and a chemical reconstruction of skin scars (CROSS) method in the treatment of acne scars: a simultaneous split‐face trial. Lasers in Surgery and Medicine 2009;41(8):545‐9. [PUBMED: 19639620] [DOI] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee DH, Choi YS, Min SU, Yoon MY, Suh DH. Comparison of a 585‐nm pulsed dye laser and a 1064‐nm Nd:YAG laser for the treatment of acne scars: a randomized split‐face clinical study. Journal of the American Academy of Dermatology 2009;60(5):801‐7. [PUBMED: 19217691] [DOI] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee JW, Kim BJ, Kim MN, Mun SK. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split‐face trial. Dermatologic Surgery 2011;37(7):931‐8. [PUBMED: 21635618] [DOI] [PubMed] [Google Scholar]
Leheta 2011 {published data only}
- Leheta T, Tawdy A, Abdel Hay R, Farid S. Percutaneous collagen induction versus full‐concentration trichloroacetic acid in the treatment of atrophic acne scars. Dermatologic Surgery 2011;37(2):207‐16. [PUBMED: 21269351] [DOI] [PubMed] [Google Scholar]
- Leheta T, Tawdy AE, Hay RA, Farid S. Percutaneous collagen induction versus full concentration trichloroacetic acid in the treatment of atrophic acne scars. 21st Annual Meeting of the European Tissue Repair Society Amsterdam Netherlands. Conference Start: 20111005 Conference End: 20111007. Wound Repair and Regeneration 2011;19(5):A70. [EMBASE: 70532581] [Google Scholar]
Leheta 2014a {published data only}
- Leheta TM, Abdel Hay RM, Garem YF. Deep peeling using phenol versus percutaneous collagen induction combined with trichloroacetic acid 20% in atrophic post‐acne scars; a randomized controlled trial. Journal of Dermatological Treatment 2014;25(2):130‐6. [PUBMED: 22397516 ] [DOI] [PubMed] [Google Scholar]
Leheta 2014b {published data only}
- Leheta TM, Abdel Hay RM, Hegazy RA, Garem YF. Do combined alternating sessions of 1540 nm nonablative fractional laser and percutaneous collagen induction with trichloroacetic acid 20% show better results than each individual modality in the treatment of atrophic acne scars? A randomized controlled trial. Journal of Dermatological Treatment 2014;25(2):137‐41. [PUBMED: 22640000] [DOI] [PubMed] [Google Scholar]
Linkner 2014 {published data only}
- Linkner RV, Jim On S, Haddican M, Singer G, Shim‐Chang H. Evaluating the efficacy of photodynamic therapy with 20% aminolevulinic acid and microdermabrasion as a combination treatment regimen for acne scarring: A split‐face, randomized, double‐blind pilot study. Journal of Clinical & Aesthetic Dermatology 2014;7(5):32‐5. [PUBMED: 24847407] [PMC free article] [PubMed] [Google Scholar]
Manuskiatti 2013 {published data only}
- Manuskiatti W, Iamphonrat T, Wanitphakdeedecha R, Eimpunth S. Comparison of fractional Er:YAG and CO2 lasers in resurfacing of atrophic acne scars in Asians. 31st Annual Conference of the American Society for Laser Medicine and Surgery, ASLMS 2011 Grapevine, TX United States. Conference Start: 20110330 Conference End: 20110403. Lasers in Surgery and Medicine 2011;43:938‐939. [EMBASE: 70640237] [Google Scholar]
- Manuskiatti W, Iamphonrat T, Wanitphakdeedecha R, Eimpunth S. Comparison of fractional erbium‐doped yttrium aluminum garnet and carbon dioxide lasers in resurfacing of atrophic acne scars in Asians. Dermatologic Surgery 2013;39(1 Pt 1):111‐20. [EMBASE: 23205717] [DOI] [PubMed] [Google Scholar]
Min 2009 {published data only}
- Min SU, Choi YS, Lee DH, Yoon MY, Suh DH. Comparison of a long‐pulse Nd:YAG laser and a combined 585/1,064‐nm laser for the treatment of acne scars: a randomized split‐face clinical study. Dermatologic Surgery 2009;35(11):1720‐7. [PUBMED: 19250299] [DOI] [PubMed] [Google Scholar]
Munavalli 2013 {published data only}
- Munavalli GS, Smith S, Maslowski JM, Weiss RA. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi‐site, prospective, double blind, placebo‐controlled clinical trial. Dermatologic Surgery 2013;39(8):1226‐36. [PUBMED: 23566237] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rongsaard 2014 {published data only}
- Rongsaard N, Rummaneethorn P. Comparison of a fractional bipolar radiofrequency device and a fractional erbium‐doped glass 1,550‐nm device for the treatment of atrophic acne scars: a randomized split‐face clinical study. Dermatologic Surgery 2014;40(1):14‐21. [PUBMED: 24267397] [DOI] [PubMed] [Google Scholar]
Sage 2011 {published data only}
- Sage RJ, Lopiccolo MC, Liu A, Mahmoud BH, Tierney EP, Kouba DJ. Subcuticular incision versus naturally sourced porcine collagen filler for acne scars: a randomized split‐face comparison. Dermatologic Surgery 2011;37(4):426‐31. [PUBMED: 21388487] [DOI] [PubMed] [Google Scholar]
Tanzi 2004 {published data only}
- Tanzi EL, Alster TS. Comparison of a 1450‐nm diode laser and a 1320‐nm Nd:YAG laser in the treatment of atrophic facial scars: a prospective clinical and histologic study. Dermatologic Surgery 2004;30(2 Pt 1):152‐7. [PUBMED: 14756642] [DOI] [PubMed] [Google Scholar]
Zhang 2013 {published data only}
- Zhang Z, Fei Y, Chen X, Lu W, Chen J. Comparison of a fractional microplasma radio frequency technology and carbon dioxide fractional laser for the treatment of atrophic acne scars: a randomized split‐face clinical study. Dermatologic Surgery 2013;39(4):559‐66. [PUBMED: 23379344] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahmed 2014 {published data only}
- Ahmed R, Mohammed G, Ismail N, Elakhras A. Randomized clinical trial of CO₂ LASER pinpoint irradiation technique versus chemical reconstruction of skin scars (CROSS) in treating ice pick acne scars. Journal of Cosmetic and Laser Therapy 2014;16(1):8‐13. [EMBASE: 2014075366] [DOI] [PubMed] [Google Scholar]
Alexis 2011 {published data only}
- Alexis A, Coley M, Alam M, Luke J, Shah S, Argobi Y. A prospective randomized split‐face comparison study of non‐ablative fractional laser resurfacing in the treatment of acne scarring in fitzpatrick skin phototypes IV‐VI. 31st Annual Conference of the American Society for Laser Medicine and Surgery, ASLMS 2011 Grapevine, TX United States. Conference Start: 20110330 Conference End: 20110403. Lasers in Surgery and Medicine 2011;43:939. [EMBASE: 70640238] [Google Scholar]
Alster 1996 {published data only}
- Alster TS, McMeekin TO. Improvement of facial acne scars by the 585 nm flashlamp‐pumped pulsed dye laser. Journal of the American Academy of Dermatology 1996;35(1):79‐81. [EMBASE: 1996211032] [DOI] [PubMed] [Google Scholar]
Azzam 2013 {published data only}
- Azzam OA, Atta AT, Sobhi RM, Mostafa PIN. Fractional CO2 laser treatment vs autologous fat transfer in the treatment of acne scars: A comparative study. Journal of Drugs in Dermatology 2013;12(1):e7‐e13. [EMBASE: 2013090597] [PubMed] [Google Scholar]
Balighi 2008 {published data only}
- Balighi K, Robati RM, Moslehi H, Robati AM. Subcision in acne scar with and without subdermal implant: a clinical trial. Journal of the European Academy of Dermatology & Venereology 2008;22(6):707‐11. [EMBASE: 2008238716] [DOI] [PubMed] [Google Scholar]
Bjørn 2014 {published data only}
- Bjørn M, Stausbøl‐Grøn B, Braae Olesen A, Hedelund L. Treatment of acne scars with fractional CO2 laser at 1‐month versus 3‐month intervals: An intra‐individual randomized controlled trial. Lasers in Surgery and Medicine 2014;46(2):89‐93. [EMBASE: 2014128952] [DOI] [PubMed] [Google Scholar]
Dreno 2007a {published data only}
- Dreno B, Katsambas A, Pelfini C, Plantier D, Jancovici E, Ribet V, et al. Combined 0.1% retinaldehyde/ 6% glycolic acid cream in prophylaxis and treatment of acne scarring. Dermatology 2007;214(3):260‐7. [PUBMED: 17377389] [DOI] [PubMed] [Google Scholar]
Gadkari 2014 {published data only}
- Gadkari R, Nayak C. A split‐face comparative study to evaluate efficacy of combined subcision and dermaroller against combined subcision and cryoroller in treatment of acne scars. Journal of Cosmetic Dermatology 2014;13(1):38‐43. [PUBMED: 24641604] [DOI] [PubMed] [Google Scholar]
Gawdat 2014 {published data only}
- Gawdat HI, Hegazy RA, Fawzy MM, Fathy M. Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars.[Erratum appears in Dermatol Surg. 2014 May;40(5):601]. Dermatologic Surgery 2014;40(2):152‐61. [PUBMED: 24354616] [DOI] [PubMed] [Google Scholar]
Goldman 1999 {published data only}
- Goldman MP, Manuskiatti W. Combined laser resurfacing with the 950‐microsec pulsed CO2 + Er:YAG lasers. Dermatologic Surgery 1999;25(3):160‐3. [PUBMED: 10193959] [DOI] [PubMed] [Google Scholar]
Jung 2010 {published data only}
- Jung JY, Lee JH, Ryu DJ, Lee SJ, Bang D, Cho SB. Lower‐fluence, higher‐density versus higher‐fluence, lower‐density treatment with a 10,600‐nm carbon dioxide fractional laser system: A split‐face, evaluator‐blinded study. Dermatologic Surgery 2010;36(12):2022‐9. [EMBASE: 2010679252] [DOI] [PubMed] [Google Scholar]
Kim 2009a {published data only}
- Kim S, Cho KH. Clinical trial of dual treatment with an ablative fractional laser and a nonablative laser for the treatment of acne scars in Asian patients. Dermatologic Surgery 2009;35(7):1089‐98. [PUBMED: 19438689] [DOI] [PubMed] [Google Scholar]
Laubach 2008 {published data only}
- Laubach H‐J. Does penetration depth matter for fractional photothermolysis? Preliminary results of a prospective, randomized split‐face study using non‐ablative fractional photothermolysis in the treatment of acne scars [La profondeur de penetration a‐t‐elle de l'importance dans la photothermolyse fractionnelle? Resultats preliminaires d'une etude prospective et randomisee par demi‐visage avec la photothermolyse fractionnelle non ablative dans le traitement des cicatrices d'acne]. Nouvelles Dermatologiques 2008;27(5 Spec Iss):45. [EMBASE: 2008287801] [Google Scholar]
Laubach 2009 {published data only}
- Laubach H‐J, Manstein D, Zurakowski D, Bayer H, Peukert N, Becke D, et al. Non‐ablative fractional photothermolysis of acne scars: Effects of energy distribution and penetration depth. 29th Annual Conference of the American Society for Laser Medicine and Surgery National Harbor, MD United States. Conference Start: 20090401 Conference End: 20090405. Lasers in Surgery and Medicine 2009;41:31. [EMBASE: 70332706] [Google Scholar]
Lee 2014 {published data only}
- Lee SJ, Kang JM, Chung WS, Kim YK, Kim HS. Ablative non‐fractional lasers for atrophic facial acne scars: a new modality of erbium:YAG laser resurfacing in Asians. Lasers in Medical Science 2014;29(2):615‐9. [EMBASE: 2014211982] [DOI] [PubMed] [Google Scholar]
Mohammed 2013 {published data only}
- Mohammed G. Randomized clinical trial of CO2 laser pinpoint irradiation technique with/without needling for ice pick acne scars. Journal of Cosmetic and Laser Therapy 2013;15(3):177‐82. [EMBASE: 2013337358] [DOI] [PubMed] [Google Scholar]
Nofal 2014 {published data only}
- Nofal E, Helmy A, Nofal A, Alakad R, Nasr M. Platelet‐rich plasma versus CROSS technique with 100% trichloroacetic acid versus combined skin needling and platelet rich plasma in the treatment of atrophic acne scars: a comparative study. Dermatologic Surgery 2014;40(8):864‐73. [PUBMED: 25006854] [DOI] [PubMed] [Google Scholar]
Sharad 2011 {published data only}
- Sharad J. Combination of microneedling and glycolic acid peels for the treatment of acne scars in dark skin. Journal of Cosmetic Dermatology 2011;10(4):317‐23. [PUBMED: 22151943] [DOI] [PubMed] [Google Scholar]
Srivastava 2009 {published data only}
- Mahmoud BH, Srivastava D, Janiga JJ, Yang JJ, Lim HW, Ozog DM. Safety and efficacy of erbium‐doped yttrium aluminum garnet fractionated laser for treatment of acne scars in type IV to VI skin. Dermatologic Surgery 2010;36(5):602‐9. [PUBMED: 20384757] [DOI] [PubMed] [Google Scholar]
- Srivastava D, Mahmoud B, Ozog D, Lim H, Janiga J. The safety and efficacy of fractional laser treatments for acne scars in Fitzpatrick skin types IV to VI. 67th Annual Meeting of the American Academy of Dermatology, AAD San Francisco, CA United States. Conference Start: 20090306 Conference End: 20090310. Journal of the American Academy of Dermatology 2009;60(3 Suppl 1):AB197. [EMBASE: 70142499] [Google Scholar]
Tanghetti 2013 {published data only}
- Tanghetti E, Tanghetti M. Is deeper better: a prospective study of deep vs superficial non‐ablative fractional laser treatment of acne scars and photo‐aging. 33rd Annual Conference of the American Society for Laser Medicine and Surgery, ASLMS 2013 Boston, MA United States. Conference Start: 20130403 Conference End: 20130407. Lasers in Surgery and Medicine 2013;45:4. [EMBASE: 71033948] [Google Scholar]
Vejjabhinanta 2014 {published data only}
- Vejjabhinanta V, Wanitphakdeedecha R, Limtanyakul P, Manuskiatti W. The efficacy in treatment of facial atrophic acne scars in Asians with a fractional radiofrequency microneedle system. Journal of the European Academy of Dermatology and Venereology : JEADV 2014;28(9):1219‐25. [PUBMED: 25158223] [DOI] [PubMed] [Google Scholar]
Yaghmai 2005 {published data only}
- Yaghmai D, Garden JM, Bakus AD, Massa MC. Comparison of a 1,064 nm laser and a 1,320 nm laser for the nonablative treatment of acne scars. Dermatologic Surgery 2005;31(8 Pt 1):903‐9. [PUBMED: 16042934] [DOI] [PubMed] [Google Scholar]
Yuan 2014 {published data only}
- Yuan XH, Zhong SX, Li SS. Comparison study of fractional carbon dioxide laser resurfacing using different fluences and densities for acne scars in Asians: A randomized split‐face trial. Dermatologic Surgery 2014;40(5):545‐52. [PUBMED: 24645970] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Dadzie 2006 {published data only}
- Dadzie O. A pilot study to assess the efficacy of autologous fibroblasts treatment in acne scarring (Abstract P‐49). British Association of Dermatologists 86th Annual Meeting. British Journal of Dermatology 2006;155:43. [Google Scholar]
Dreno 2003 {published data only}
- Dreno B. Effect of Diacneal® in the prevention of acne scarring and pigmentation: a double blind study including quality of life evaluation. Abstract SAT5‐4 The 12th Congress of the European Academy of Dermatology and Venereology. Barcelona, Spain 15‐18th October. Journal of the European Academy of Dermatology and Venereology : JEADV 2003;17(Suppl 3):431. [Google Scholar]
Ghaly 2012 {published data only}
- Ghaly N, Allam A. Comparative study of 1540‐nm nonablative fractional erbium laser versus 2940‐nm ablative erbium: YAG laser in resurfacing of postacne scarring. 70th Annual Meeting of the American Academy of Dermatology San Diego, CA United States. Conference Start: 20120316 Conference End: 20120320. Journal of the American Academy of Dermatology 2012;66(4 Suppl 1):AB25. [EMBASE: 70703951] [Google Scholar]
Grimes 2014 {published data only}
- Grimes P, Smith S. Results from a pivotal study on ArteFill for treating acne scars. 72nd Annual Meeting of the American Academy of Dermatology Denver, CO United States. Conference Start: 20140321 Conference End: 20140325. Journal of the American Academy of Dermatology 2014;70(5 Suppl 1):AB20. [EMBASE: 71390176] [Google Scholar]
Munavalli 2011 {published data only}
- Munavalli G, Anderson R. Fractional bipolar radiofrequency delivered through a vacuum‐assisted, microneedle array for the treatment of acne scarring‐a pilot study. 31st Annual Conference of the American Society for Laser Medicine and Surgery, ASLMS 2011 Grapevine, TX United States. Conference Start: 20110330 Conference End: 20110403. Lasers in Surgery and Medicine 2011;43:925‐6. [EMBASE: 70640200] [Google Scholar]
Qian 2014 {published data only}
- Qian L, Gao L, Liu B, Gao T, Wang G. A randomized controlled clinical trial of 10600 nm CO2 fractional laser and 2940 nm erbium fractional laser in the treatment of acne scars. 3rd Eastern Asia Dermatology Congress Jeju South Korea. Conference Start: 20140924 Conference End: 20140926. Journal of Dermatology 2014;41:69. [EMBASE: 71669032] [Google Scholar]
Sachsenberg‐Studer 2002 {published data only}
- Sachsenberg‐Studer EM, Mengeaud V, Dupuy P, Kaufmann R. Pre and post treatment of laser skin resurfacing: comparison of retinaldehyde and retinoic acid. Annales de Dermatologie et de Venereologie 2002;129(Suppl 1 Pt 2):1S614. [Google Scholar]
Sarkate 2015 {published data only}
- Sarkate A, Ghate S, Dhurat R. Split‐face placebo‐controlled study to evaluate the efficacy of type A botulinum toxin in postacne facial scars. 95th Annual Meeting of the British Association of Dermatologists Manchester United Kingdom. Conference Start: 20150707 Conference End: 20150709. British Journal of Dermatology 2015;173:68. [EMBASE: 71969514] [Google Scholar]
Sarnoff 2012 {published data only}
- Sarnoff D, Gotkin R. Evaluation of the safety and efficacy of dual treatment with an ablative fractional CO2 laser and a non‐ablative 1440nm Nd:YAG laser for atrophic facial acne scars. 32nd Annual Conference of the American Society for Laser Medicine and Surgery, ASLMS 2012 Kissimmee, FL United States. Conference Start: 20120418 Conference End: 20120422. Lasers in Surgery and Medicine 2012;44:11‐2. [EMBASE: 70716660] [Google Scholar]
References to ongoing studies
NCT02216864 {unpublished data only}
- NCT02216864. Effect of multiple subcisions on rolling a scars. clinicaltrials.gov/ct2/show/study/NCT02216864 (accessed 30 December 2015).
NCT02643628 {unpublished data only}
- NCT02643628. A pilot study to evaluate the safety and effectiveness of microneedling and bellafill to treat facial acne scars. clinicaltrials.gov/ct2/show/study/NCT02643628 (accessed 30 December 2015).
Additional references
Alam 2005
- Alam M, Omura N, Kaminer MS. Subcision for acne scarring: technique and outcomes in 40 patients. Dermatologic Surgery 2005;31(3):310‐7; 317. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Alexiades‐Armenakas 2008
- Alexiades‐Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. Journal of the American Academy of Dermatology 2008;58(5):719‐37; 738‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Alster 2003
- Alster TS, Tanzi EL. Hypertrophic scars and keloids: etiology and management. American Journal of Clinical Dermatology 2003;4(4):235‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arno 2014
- Arno AI, Gauglitz GG, Barret JP, Jeschke MG. Up‐to‐date approach to manage keloids and hypertrophic scars: a useful guide. Burns 2014;40(7):1255‐66. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Basta‐Juzbasic 2010
- Basta‐Juzbasic A. Current therapeutic approach to acne scars. Acta Dermatovenerologica Croatica 2010;18(3):171‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Brown 2009
- Brown JJ, Bayat A. Genetic susceptibility to raised dermal scarring. British Journal of Dermatology 2009;161(1):8‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cao 2015
- Cao H, Yang G, Wang Y, Liu J P, Smith CA, Luo H, et al. Complementary therapies for acne vulgaris. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD009436.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collier 2007
- Collier CN, Harper JC, Cafardi JA, Cantrell WC, Wang W, Foster KW, et al. The prevalence of acne in adults 20 years and older. Journal of the American Academy of Dermatology 2007;58(1):56‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Conrado 2010
- Conrado LA, Hounie AG, Diniz JB, Fossaluza V, Torres AR, Miguel EC, et al. Body dysmorphic disorder among dermatologic patients: prevalence and clinical features. Journal of the American Academy of Dermatology 2010;63(2):235‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dalgard 2009
- Dalgard F, Gieler U, Holm JO, Bjertness E, Hauser S. Self‐esteem and body satisfaction among late adolescents with acne: results from a population survey. Journal of the American Academy of Dermatology 2009;59(5):746‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dreno 2007
- Dreno B, Khammari A, Orain N, Noray C, Mérial‐Kieny C, Méry S, et al. ECCA grading scale: an original validated acne scar grading scale for clinical practice in dermatology. Dermatology 2007;214(1):46‐51. [PUBMED: 17191047] [DOI] [PubMed] [Google Scholar]
Duman 2015
- Duman H, Topal IO, Kocaturk E, Duman MA. Evaluation of anxiety, depression, and quality of life in patients with acne vulgaris, and quality of life in their families. Dermatologica Sinica In press. [DOI: 10.1016/j.dsi.2015.07.002] [DOI]
Fabbrocini 2009
- Fabbrocini G, Fardella N, Monfrecola A, Proietti I, Innocenzi D. Acne scarring treatment using skin needling. Clinical and Experimental Dermatology 2009;34(8):874‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fabbrocini 2010
- Fabbrocini G, Annunziata MC, D'Arco V, Vita V, Lodi G, Mauriello MC, et al. Acne scars: pathogenesis, classification and treatment. Dermatology Research and Practice 2010;2010:893080. [PUBMED: 20981308] [DOI] [PMC free article] [PubMed] [Google Scholar]
Finlay 2013
- Finlay AY, Torres V, Kang S, Bettoli V, Dreno B, Goh CL, et al. Classification of acne scars is difficult even for acne experts. Journal of the European Academy of Dermatology and Venereology 2013;27(3):391‐3. [PUBMED: 22329412] [DOI] [PubMed] [Google Scholar]
Garg 2008
- Garg VK, Sinha S, Sarkar R. Glycolic acid peels versus salicylic‐mandelic acid peels in active acne vulgaris and post‐acne scarring and hyperpigmentation: a comparative study. Dermatologic Surgery 2008;35(1):59‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garg 2014
- Garg S, Baveja S. Combination therapy in the management of atrophic acne scars. Journal of Cutaneous and Aesthetic Surgery 2014;7(1):18‐23. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gauglitz 2011
- Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Molecular Medicine 2011;17(1‐2):113‐25. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goel 2011
- Goel A, Krupashankar DS, Aurangabadkar S, Nischal KC, Omprakash HM, Mysore V. Fractional lasers in dermatology‐‐current status and recommendations. Indian Journal of Dermatology, Venereology and Leprology 2011;77(3):369‐79. [DOI: 10.4103/0378-6323.79732] [DOI] [PubMed] [Google Scholar]
Gold 2014
- Gold MH, McGuire M, Mustoe TA, Pusic A, Sachdev M, Waibel J, et al. Updated international clinical recommendations on scar management: part 2‐‐algorithms for scar prevention and treatment. Dermatologic Surgery 2014;40(8):825‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goodman 2006a
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatologic Surgery 2006;32(12):1458‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goodman 2006b
- Goodman GJ, Baron JA. Postacne scarring‐‐a quantitative global scarring grading system. Journal of Cosmetic Dermatology 2006;5(1):48‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goodman 2007
- Goodman GJ, Baron JA. The management of postacne scarring. Dermatologic Surgery 2007;33(10):1175‐88. [EMBASE: 2007481881] [DOI] [PubMed] [Google Scholar]
Goodman 2014
- Goodman GJ. Treatment of acne scarring. In: Zouboulis Christos C, Katsambas Andreas D, Kligman Albert M editor(s). Pathogenesis and Treatment of Acne and Rosacea. Heidelberg: Springer Berlin, 2014:527‐36. [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool. Version 2015. McMaster University (developed by Evidence Prime, Inc.), 2015.
Grevelink 1998
- Grevelink JM, White VR. Concurrent use of laser skin resurfacing and punch excision in the treatment of facial acne scarring. Dermatologic Surgery 1998;24(5):527‐30. [EMBASE: 1998165350] [DOI] [PubMed] [Google Scholar]
Gupta 2013
- Gupta R, Huynh M, Ginsburg IH. Body dysmorphic disorder. Seminars in Cutaneous Medicine and Surgery 2013;32(2):78‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hay 2014
- Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. Journal of Investigative Dermatology 2014;134(6):1527‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holland 2004
- Holland DB, Jeremy AH, Roberts SG, Seukeran DC, Layton AM, Cunliffe WJ. Inflammation in acne scarring: a comparison of the responses in lesions from patients prone and not prone to scar. British Journal of Dermatology 2004;150(1):72‐81. [PUBMED: 14746619] [DOI] [PubMed] [Google Scholar]
Ingram 2015
- Ingram JR, Woo PN, Chua SL, Ormerod AD, Desai N, Kai AC, et al. Interventions for hidradenitis suppurativa. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD010081.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacob 2001
- Jacob CI, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. Journal of the American Academy of Dermatology 2001;45(1):109–17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Landau 2007
- Landau M. Cardiac complications in deep chemical peels. Dermatologic Surgery 2007;33(2):190‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Layton 1994
- Layton AM, Henderson CA, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clinical and Experimental Dermatology 1994;19(4):303‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Layton 2015
- Layton A, Eady EA, Peat M, Whitehouse H, Levell N, Ridd M, et al. Identifying acne treatment uncertainties via a James Lind Alliance Priority Setting Partnership. BMJ Open 2015;5(7):e008085. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Levy 2012
- Levy LL, Zeichner JA. Management of acne scarring, part II: a comparative review of non‐laser‐based, minimally invasive approaches. American Journal of Clinical Dermatology 2012;13(5):331‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Maibach 2011
- Maibach HI, Gorouhi F. Evidence Based Dermatology. 2. Shelton, CT: People's Medical Publishing House, 2011. [1607950391] [Google Scholar]
Mutalik 2005
- Mutalik S. Treatment of keloids and hypertrophic scars. Indian Journal of Dermatology, Venereology and Leprology 2005;71(1):3‐8. [EMBASE: 2005117802] [DOI] [PubMed] [Google Scholar]
Nankervis 2015
- Nankervis H, Pynn EV, Boyle RJ, Rushton L, Williams HC, Hewson DM, et al. House dust mite reduction and avoidance measures for treating eczema. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD008426.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ong 2012
- Ong MW, Bashir SJ. Fractional laser resurfacing for acne scars: a review. British Journal of Dermatology 2012;166(6):1160‐9. [PUBMED: 22296284] [DOI] [PubMed] [Google Scholar]
Patel 2010
- Patel MJ, Antony A, Do T, Hinds G, Sachs D, J Voorhees, et al. Atrophic acne scars may arise from both inflammatory and non‐inflammatory acne lesions. 2010 Annual Meeting of the Society for Investigative Dermatology Atlanta, GA United States. Conference Start: 20100505 Conference End: 20100508. Journal of Investigative Dermatology 2010;130(Suppl 1s):S58. [EMBASE: 70123028] [Google Scholar]
Purvis 2006
- Purvis D, Robinson E, Merry S, Watson P. Acne, anxiety, depression and suicide in teenagers: a cross‐sectional survey of New Zealand secondary school students. Journal of Paediatrics and Child Health 2006;42(12):793‐6. [PUBMED: 17096715] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 2013
- Robinson KA, Akinyede O, Dutta T, Sawin VI, Li T, Spencer MR, et al. Framework for Determining Research Gaps During Systematic Review: Evaluation 2013. www.ncbi.nlm.nih.gov/books/NBK126708/ (accessed 2 November 2015). [PUBMED: 23487868] [PubMed]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, (editors). GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. www.guidelinedevelopment.org/handbook (accessed 2 November 2015).
Sobanko 2012
- Sobanko JF, Alster TS. Management of acne scarring, part I: a comparative review of laser surgical approaches. American Journal of Clinical Dermatology 2012;13(5):319‐30. [PUBMED: 22612738] [DOI] [PubMed] [Google Scholar]
Strauss 2007
- Strauss JS, Krowchuk DP, Leyden JJ, Lucky AW, Shalita AR, Siegfried EC, et al. Guidelines of care for acne vulgaris management. Journal of the American Academy of Dermatology 2007;56(4):651‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sánchez Viera 2015
- Sánchez Viera M. Management of acne scars: fulfilling our duty of care for patients. British Journal of Dermatology 2015;172(Suppl 1):47‐51. [PUBMED: 25597636] [DOI] [PubMed] [Google Scholar]
Williams 2012
- Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet 2012;379(9813):361‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Abdel Hay 2015
- Abdel Hay R, Shalaby K, Zaher H, Hafez V, Chi C‐C, Dimitri S, et al. Interventions for acne scars. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011946] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jordan 2000
- Jordan R, Cummins CCL, Burls A, Seukeran DDC. Laser resurfacing for facial acne scars. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD001866] [DOI] [PubMed] [Google Scholar]


