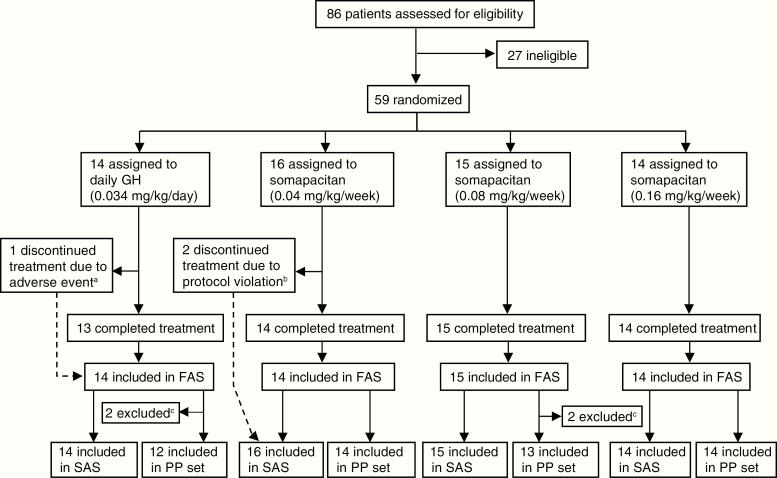
Figure 2.
Trial profile. The FAS was to contain all randomly assigned children who received at least 1 dose of randomized treatment. Only in exceptional cases, such as random assignment in error, could children be excluded from the FAS. PP set: children from the FAS who did not violate any inclusion/exclusion criteria and used the randomized treatment for at least 22 weeks (for children receiving somapacitan) or 154 days (for children receiving daily GH) during the main trial period. SAS: all randomly assigned children who received at least 1 dose of randomized treatment. aOne child in the daily GH group discontinued because of a nonserious adverse event (mild drug hypersensitivity), which was deemed probably related to treatment. bOne child was withdrawn by parents who declined further participation, and the other had a prior history or presence of malignancy and/or intracranial tumor. cOne child in the daily GH arm was excluded from the PP set because of not enough exposure (the patient referred to in footnote a). The other 3 children were excluded because of a violation of the height velocity (HV) inclusion criterion. FAS, full analysis set; GH indicates growth hormone; PP, per protocol analysis set; SAS, safety analysis set.