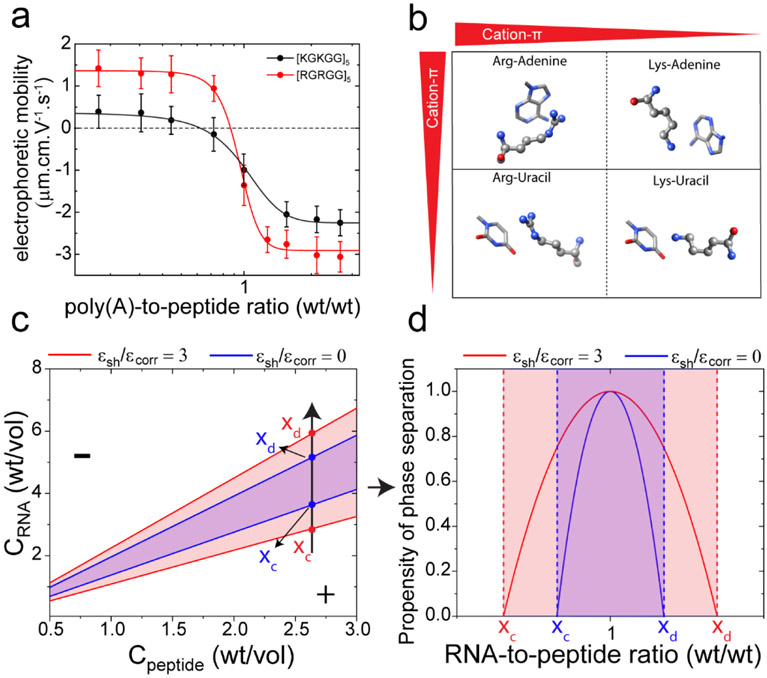
Figure 3: Short-range cation-π attraction widens the LLPS regime of peptide-RNA complexes.
a) Electrophoretic mobility of poly(A)-[RGRGG]5 and poly(A)-[KGKGG]5 complexes as a function of poly(A)-to-peptide ratio. Solid lines represent fits to a two-state model. b) Schematics showing cation-π interactions between Arg/Lys-nucleobases and their relative strengths. c) Simulated phase diagrams showing widening of the LLPS regimes (red vs. blue) due to short-range attraction. ϵcorr is the correlation energy that represents an enthalpic driving force of LLPS in RLC model and ϵsh is the short-range inter-complex attraction. (d) Propensity of phase separation calculated from equation-9 in SI-note 1. The two curves here represent the values of the RNA-to-peptide ratio at which the inter-complex attraction leading to phase separation is favorable. They correspond to the absolute value of the relative energy loss due to phase separation, which is maximal at RNA-to-peptide ratio of 1.0. In regions outside the boundary marked by xc and xd, phase separation is not favorable. Parameters used for generating (c) and (d) are: RNA charge Q=2200, peptide charge q = 10, correlation energy ϵcorr=1000. See SI Note-1 for further details.