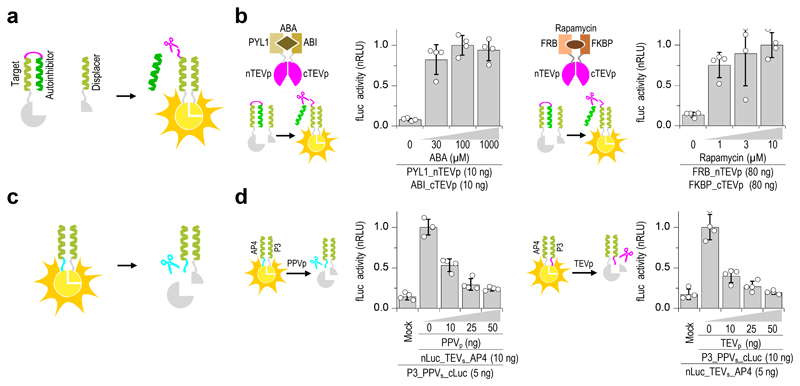
Figure 2. Design of proteolytic cleavage-responsive coiled-coil (CC) interaction modules.
(a) Design of the proteolytic cleavage-responsive CC rearrangement reconstituting the functional split protein. Upon linker cleavage, an autoinhibitory coil is replaced by a displacer segment with higher binding affinity to reconstitute the split effector/reporter. (b) Abscisic acid (ABA) and rapamycin inducible activation was demonstrated in HEK293T cells measured 30 min after induction with indicated concentration of ABA or rapamycin. (c) Design of a proteolytic cleavage-inactivated module (logical negation). A protease cleavage site is introduced between the CC-forming segments and split effector/reporter domain. After cleavage of the linker, split luciferase dissociates. (d) Decrease in luciferase activity upon co-transfection of logical negation functions with plasmids coding for specific proteases. Values in (b), and (d) are the mean of four cell cultures ± (s.d.) and representative of two independent experiments. Transfection plasmid mixtures are listed in Supplementary Table 1.