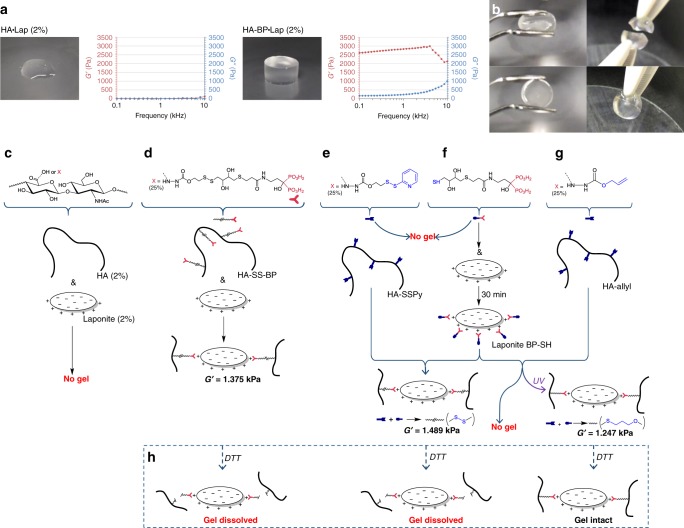
Fig. 1. Laponite bisphosphonate interactions allow physical cross-linking of hydrogels.
Laponite addition (2% wt. vol.) to a bisphosphonate (BP) functionalized (DS = 25%) hyaluronic acid (HA) polymer (2 % wt. vol.) yields a stiff gel, while no gel is formed following addition of Laponite to HA alone (a graph displays storage (G′) and loss (G′′) moduli of gels under a frequency sweep). HA-BP•Laponite gels can be manipulated using forceps and self-heal (b). As well as achieving Laponite cross-linking via BP disulfide functionalization of the HA backbone (HA-SS-BP; c and d), thiol functionalization of the Laponite particles themselves via incubation with low molecular weight thiol-terminated BP derivatives (BP-SH) was also achieved (f). Thiol functionality of Laponite•BP-SH was confirmed via successful gelation of hyaluronic acid modified with dithiopyridyl groups (HA-SSPy) following addition of Laponite•BP-SH, but not of BP-SH derivatives alone (e). Thiol functionality of Laponite•BP-SH was also confirmed via successful photoinitiated gelation of allyl- functionalized HA in the presence of Laponite•BP-SH. No gelation of HA-allyl + Laponite•BP-SH was observed in the absence of UV exposure (g). Treatment of the formed gels with dithiothreitol (DTT) resulted in dissolution only of those nanocomposite gels where BP groups were conjugated via a labile disulfide bond (h). G′ measurements were conducted at a frequency of 1 Hz.