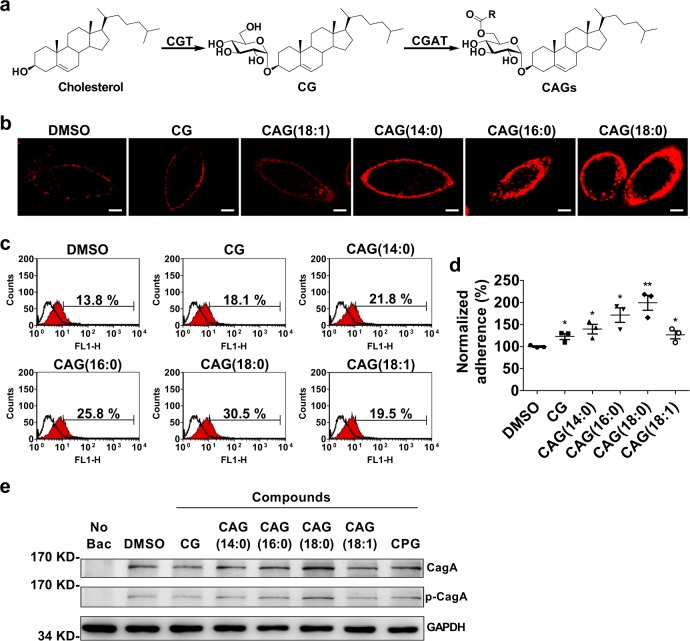
Fig. 1. CAGs of varied chain length were able to enhance H. pylori adhesion and the corresponding CagA translocation.
a Biosynthetic pathway of CAG in all H. pylori strains where cholesterol α-glucosyltransferase (CGT) and cholesteryl α-d-glucoside acyltransferase (CGAT) consecutively catalyze the reactions to yield cholesteryl α-d-glucopyranoside (CG) and CAG, respectively. The R group of CAG represents O6′-esters of different fatty acids, e.g., myristic acid (14:0), palmitic acid (16:0), stearic acid (18:0), and oleic acid (18:1). b Representative confocal images of lipid rafts clustering in the presence of CG or CAGs with different acyl chain. After AGS cells were treated with CG or CAG (as indicated) for 1 h, the lipid rafts (GM1) were then labeled with Alexa Fluor 594-conjugated cholera toxin subunit b (red fluorescence). Confocal images were collected under a Leica SP5 X inverted confocal microscope. Scale bar: 5 μm. c, d Degree of H. pylori adherence to AGS cells is dependent on the acyl chain of CAG. AGS cells were first treated with CG or CAGs (as indicated) for 1 h, infected with H. pylori strain 26695 for another 1 h, and then subjected to flow cytometry analysis. Adherence was measured as the proportion of adhered AGS cells with H. pylori (%; shown in each plot). The resulting quantitation of cell adherence was normalized in comparison with the control and shown in d. Data are shown as mean ± SD (standard deviation). All statistically significant differences are indicated with asterisks; **p < 0.01, *p < 0.05 vs. the control (n = 3). e Effects of CAGs with different acyl chains on CagA translocation and CagA tyrosine phosphorylation that was detected by immunoblotting after the co-culture of AGS cells with H. pylori 26695 as described in (c). Data sources for d are provided in Supplementary Data 1. Uncropped immunoblot images for e are provided in Supplementary Fig. 4.