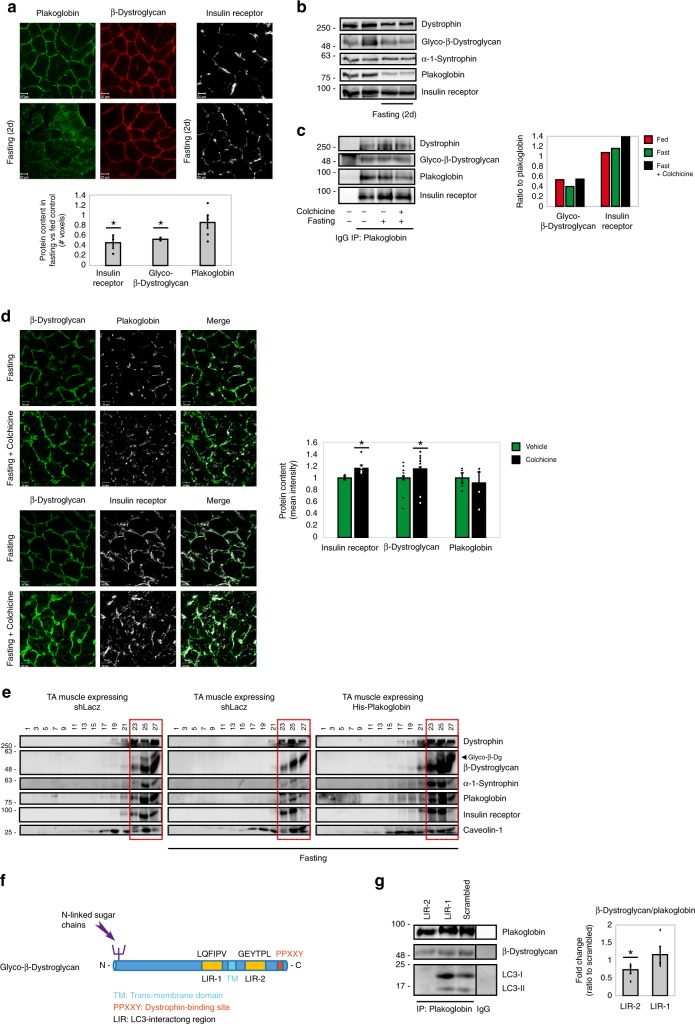
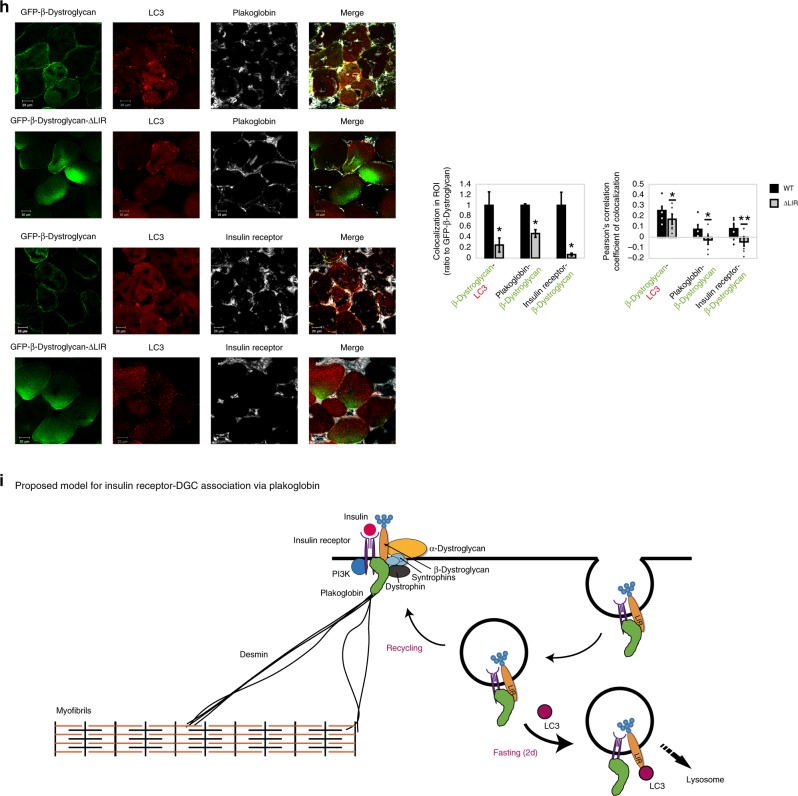
Fig. 6. Plakoglobin-DGC-insulin receptor association is regulated by autophagy.
Muscles from fed and fasted (2 days) mice are analyzed. In fasting, contralateral limbs were transfected. Data are mean ± SEM. P values by one-tailed t test. Fluorescence intensity was obtained using the Imaris software. a Top: Muscle cross-sections stained with indicated antibodies. Bar, 20 μm. n = four independent experiments. Bottom: Fluorescence intensity. n = 4. *P < 0.05 vs. fed. b Equal membrane extracts from muscles were analyzed by immunoblotting. n = two independent experiments. c Left: Plakoglobin immunoprecipitation from muscle membranes from mice treated with colchicine or vehicle was analyzed by immunoblotting. n = two independent experiments. Right: Densitometric measurement of presented blots. d Left: Muscle cross-sections from fasted mice treated with colchicine or vehicle were stained with indicated antibodies. Bar, 20 μm. n = three independent experiments. Right: Fluorescence intensity. n = 3. *P < 0.05 vs. vehicle injection. e Membranes purified from muscles expressing shLacz or 6His-plakoglobin were analyzed by glycerol gradients and immunoblot. Red rectangle marks fractions containing glycosylated-β-dystroglycan in shLacz-expressing muscles. n = four independent experiments. f Illustration of two potential LIR motifs in β-dystroglycan using iLIR database. g Left: In vitro competition assay: plakoglobin immunoprecipitation from membrane extracts from muscles of fed mice, followed by incubation in vitro with synthetic peptides corresponding to β-dystroglycan’s-LIR domains or scrambled control. β-Dystroglycan LIR-2 peptide efficiently competed with β-dystroglycan on binding to plakoglobin. Right: Densitometric measurement, β-dystroglycan to plakoglobin ratio in protein precipitates following incubation with synthetic peptides. n = four independent experiments. *P < 0.05 vs. scrambled. h Left: Cross-sections of muscles expressing GFP-β-dystroglycan or GFP-β-dystroglycan-ΔLIR from fasted mice were stained with indicated antibodies. Bar, 20 μm. n = three independent experiments. Right: Fluorescence intensity of areas of potential colocalization on the plasma membrane. n = 3. *P < 0.05 vs. GFP-β-dystroglycan. Bottom: Colocalization of indicated proteins in region of interest (region of co-occurrence, data are ratio to GFP-β-dystroglycan), and the corresponding Pearson’s correlation coefficients of colocalization. n = 7, two independent experiments. *P < 0.05 and **P < 0.005 vs. GFP-β-dystroglycan. i Proposed model: Under normal conditions, basal recycling of plakoglobin-DGC-insulin receptor co-assemblies occurs via plakoglobin masking β-dystroglycan LIR domains. During fasting, when autophagy is induced, LC3 competes with plakoglobin on binding to β-dystroglycan’s LIR domains, directing the formed vesicles to the lysosome.