Figure 1.
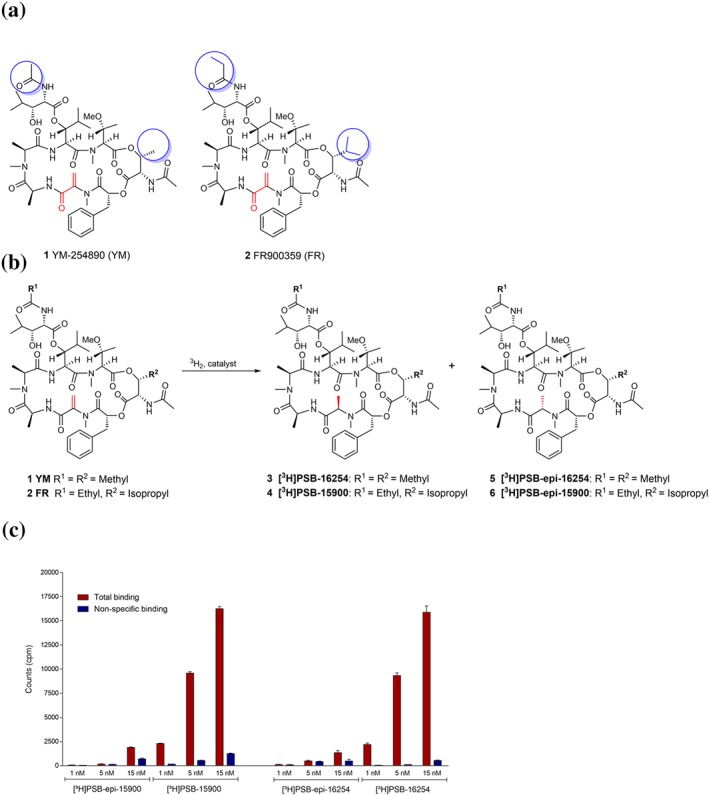
Structures, hydrogenation reaction, and preliminary binding results of Gq protein inhibitors. (a) Structures of the Gαq protein inhibitors YM and FR. Differences between YM and FR are highlighted by blue circles; reactive partial structure is highlighted in red. (b) Labelling of YM and FR by catalytic hydrogenation with tritium gas. (c) Total binding and non‐specific binding of different concentrations of the radioligands [3H]PSB‐15900 (77 Ci, 2.85 TBq·mmol−1) and its epimer [3H]PSB‐epi‐15900 (32 Ci, 1.18 TBq·mmol−1; both FR‐derived, left) and of [3H]PSB‐16254 and its epimer [3H]PSB‐epi‐16254 (both YM‐derived, right) to human platelet membrane preparations (50 μg of protein/vial). Incubation was performed at 37°C for 1 hr. Non‐specific binding was determined in the presence of 5‐μM FR900395. Values ±SD represent data from three independent experiments
