Figure 5.
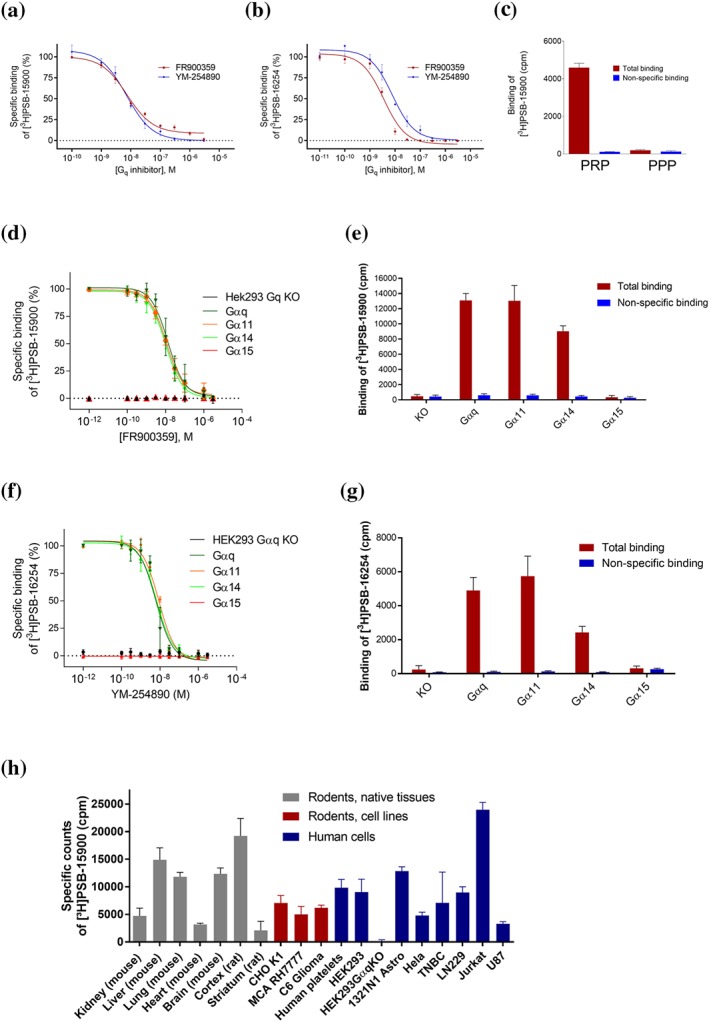
Competition binding experiments. (a, b) Competition binding experiments of FR and YM at membrane preparations of human platelets versus [3H]PSB‐15900 (5 nM) (a), and versus [3H]PSB‐16254 (5 nM) (b), performed at 37°C. Data points represent means ± SD of five independent experiments. The following pKi values were calculated: 8.39 ± 0.05 (FR vs. [3H]PSB‐15900), 8.30 ± 0.04 (YM vs. [3H]PSB‐15900), 8.60 ± 0.05 (FR vs. [3H]PSB‐16254), and 8.20 ± 0.06 (YM vs. [3H]PSB‐16254). Pseudohomologous competition (FR vs. [3H]PSB‐15900, and YM vs. [3H]PSB‐16254, respectively) yielded the following KD and Bmax values: KD for FR: 4.10 ± 0.50 nM, Bmax value 6.28 ± 1.12 pmol·mg−1 of protein; KD for YM: 6.29 ± 0.84 nM, Bmax value 6.61 ± 0.60 pmol·mg−1 of protein. (c) Binding of [3H]PSB‐15900 (5 nM) at 37°C to PRP (7.5 million platelets/vial) and binding to platelet‐poor plasma (supernatant of PRP centrifuged at 10,000 g for 30 min). Values represent means ± SD of five independent experiments. (d–g) Competition binding experiments of FR versus [3H]PSB‐15900 (5 nM) (d, e), and YM versus [3H]PSB‐16254 (5 nM) (f, g) at 37°C to membrane preparations (20 μg of protein) of stably transfected HEK‐CRISPR‐Cas9‐Gαq‐KO cells expressing different Gαq subtypes. Values represent means ± SD of five independent experiments. (h) Specific binding of [3H]PSB‐15900 (5 nM) to membrane preparations of tissues and cells (20 μg of total protein per vial). Non‐specific binding was determined by addition of 5‐μM FR. Values represent means ± SD of five independent experiments. Cell lines: CHO K1—CHO; MCA RH7777—rat liver hepatoma cells; C6 glioma—rat glioma cells; HEK293—HEK cells; 1321N1 astro—1321N1 astrocytoma cells (human); Hela—human cervix carcinoma cells; TNBC—human triple negative breast cancer cells; Jurkat—human Jurkat T‐lymphocytes; U87—human glioblastoma cells
