Figure 4.
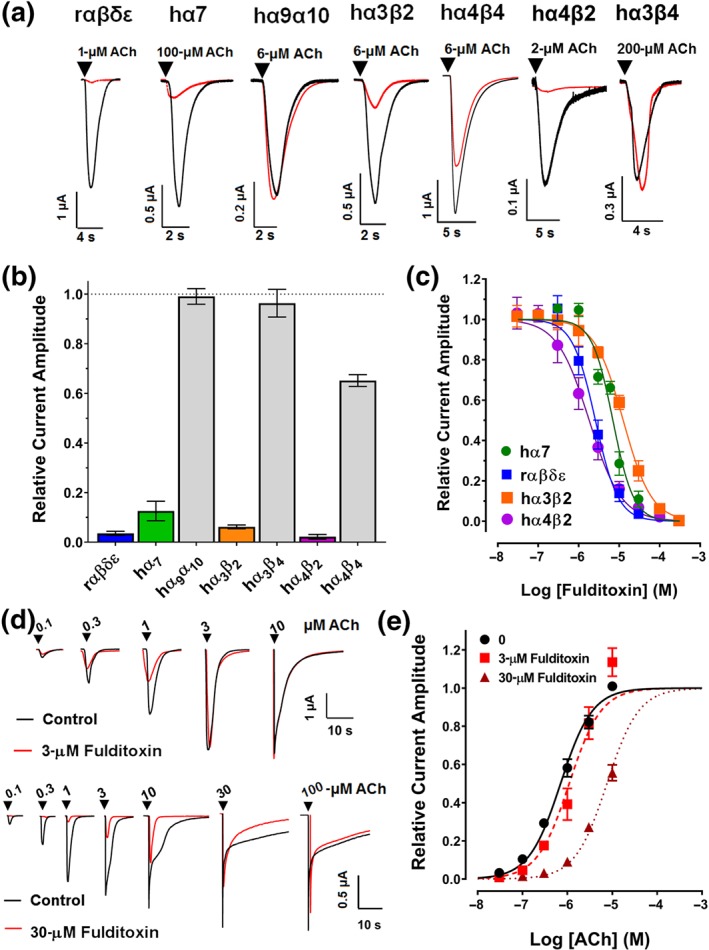
Activity of fulditoxin at nAChR subtypes expressed in Xenopus oocytes. TEVC electrophysiological characterization of synthetic fulditoxin (sFulditoxin) on nAChR subtypes expressed in Xenopus oocytes. (a) Superimposed representative ACh‐evoked currents from Xenopus oocytes expressing rαβδε, hα7, hα9α10, hα3β2, hα4β4, hα4β2, and hα3β4 nAChRs in the absence (black trace) and presence of 30‐μM sFulditoxin (red trace). For the nAChR subtypes indicated, r and h represent the species, rodent and human respectively. Whole‐cell nAChR‐mediated currents were activated by ACh at a concentration that represented the EC50 of ACh for the respective subtype expressed in the oocyte. (b) Histogram showing the effects of 30‐μM sFulditoxin on relative ACh‐evoked current amplitude mediated by rαβδε (n = 3), hα7 (n = 3), hα9α10 (n = 3), hα3β2 (n = 4), hα3β4 (n = 3), hα4β2 (n = 3), and hα4β4 (n = 4) nAChRs. (c) Concentration–response curves of sFulditoxin inhibition of selective nAChR subtypes revealed the following IC50 values for the nAChR subtypes tested: hα7 = 7.0 μM (95% CI [5.8, 8.4]; n = 3), rαβδε = 2.6 μM (95% CI [2.2, 3.1]; n = 3), hα3β2 = 12.6 μM (95% CI [11.3, 13.9]; n = 4), and hα4β2 = 1.8 μM (95% CI [1.6, 2.1; n = 4). (d) TEVC electrophysiological characterization of sFulditoxin on rodent muscle αβδε nAChRs expressed in Xenopus oocytes showing competitive antagonism of ACh binding. Representative superimposed traces of responses to varying concentrations of ACh, with or without 3‐μM sFulditoxin (top) or 30‐μM sFulditoxin (bottom). (e) Concentration–response curves obtained from the traces of ACh‐evoked responses, with or without sFulditoxin at 3 μM and 30 μM. Each data point is the mean ± SEM of three independent experiments
