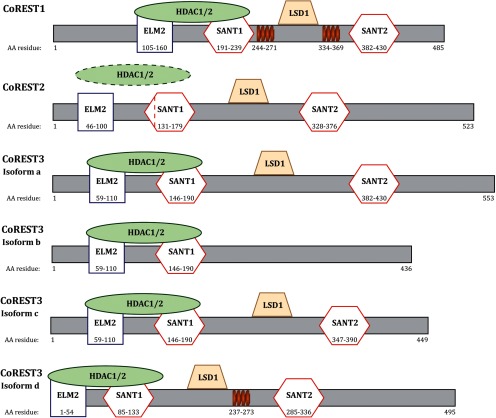
Figure 2.
Structure of the CoREST proteins. Each CoREST paralogue contains an ELM2 domain and two SANT domains. The ELM2 and SANT1 domains are responsible for recruiting HDAC1/2. CoREST2 has a non-conserved leucine residue at 165 in the SANT1 domain resulting in impaired association with HDAC1/2. The linker domain between the SANT domains is responsible for binding with LSD1. CoREST3 isoform b lacks a SANT2 domain, resulting in impaired LSD1 recruitment and is responsible for the antagonistic action of the isoform. CoREST1 and CoREST3 isoform d both contain coiled-coil domains, represented by the orange coils. Information collated via UniProt Consortium (2018) and Marchler-Bauer et al. (2017).