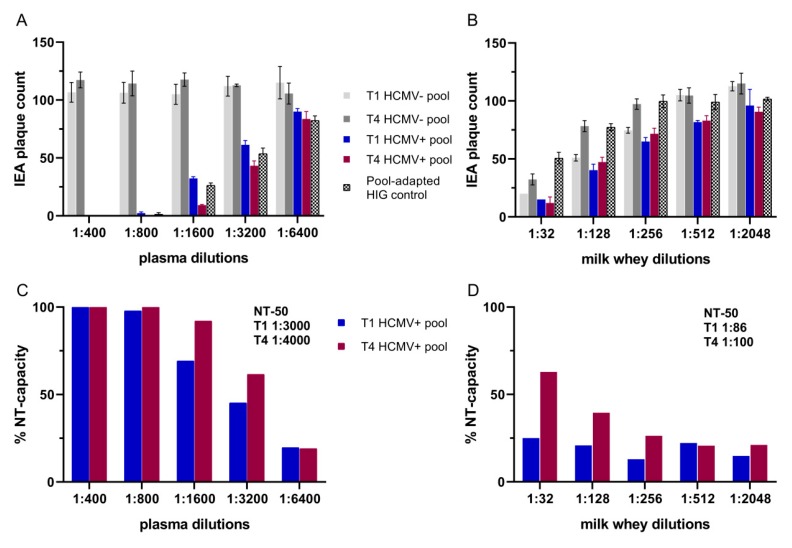
Figure 6.
Neutralization assays of HCMV-seropositive and -negative pools using ARPE-19 target cells. (A) Plasma and (B) whey immediate early antigen (IEA) plaque counts in a two-fold serial dilution (mean+SD). HCMV-IgG calibration using hyperimmunoglobulin (HIG) preparation (prediluted 1:700 to mean ECLIA values of whey and 1:6.5 for plasma values). Neutralization (NT)-capacity in (C) plasma and in (D) whey were calculated by using the HCMV-seronegative pools as 100% reference for every dilution step. NT-50 values were calculated with a Probit analysis of the seropositive pool with mean values of the seronegative pools as reference.