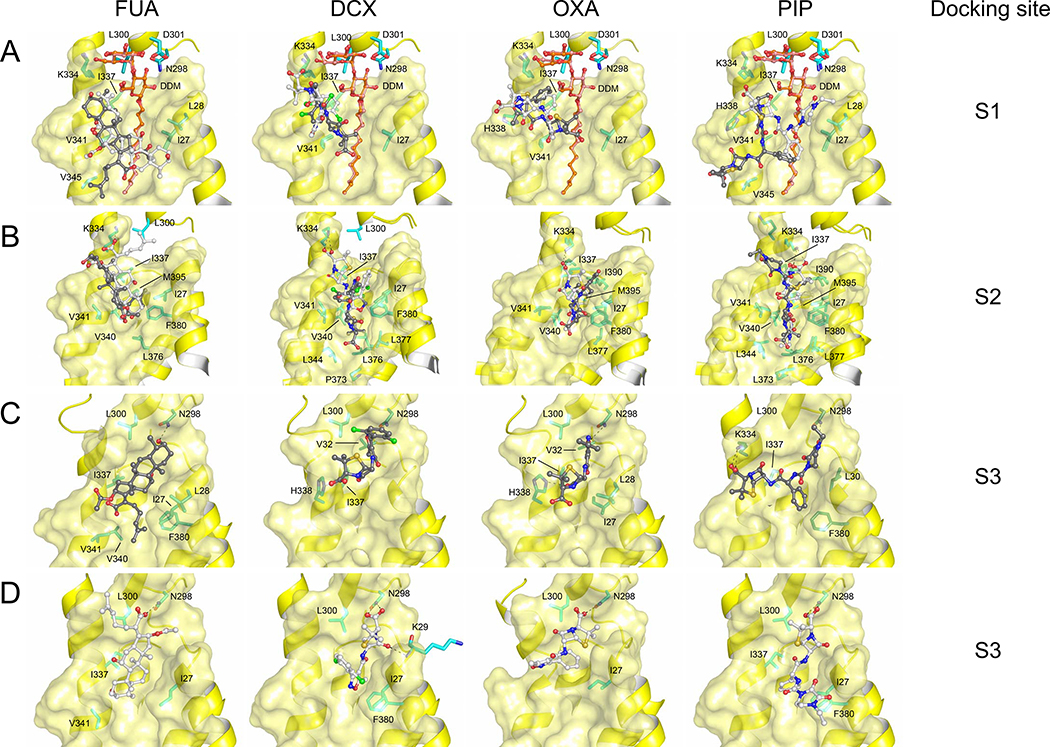
Figure 1. Potential binding conformations of FUA and carboxylated β-lactams (DCX, OXA and PIP) at the S1, S2, and S3 binding sites.
Two major docking poses were selected for all sites and depicted as light and dark gray sticks. Both (A) S1 binding sites, and (B) S2 binding sites, are presented as overlay of the two selected binding modes. (C) S3 binding sites where carboxyl groups of FUA, DCX, OXA and PIP are oriented away from the PN2 subdomain. (D) S3 binding sites where carboxyl groups of FUA, DCX, OXA and PIP are oriented towards the PN2 subdomain. The highest affinity pose of FUA where the carboxyl moiety is oriented towards the periplasm (A, light gray), is consistent with the X-ray data [18] (Fig. S1B, RMSD ~1 Å). Cartoon and surface representation are colored in yellow. DDM molecules are shown as stick models (carbon = orange oxygen = red). Drug molecules are depicted as ball and stick (carbon = black or white; oxygen = red; nitrogen = blue; chlorine = green; sulfur = yellow). Residues are depicted as stick models (carbon = cyan or green).