Figure 7. Platelet agonists activate ASK1 via a ROS independent mechanism.
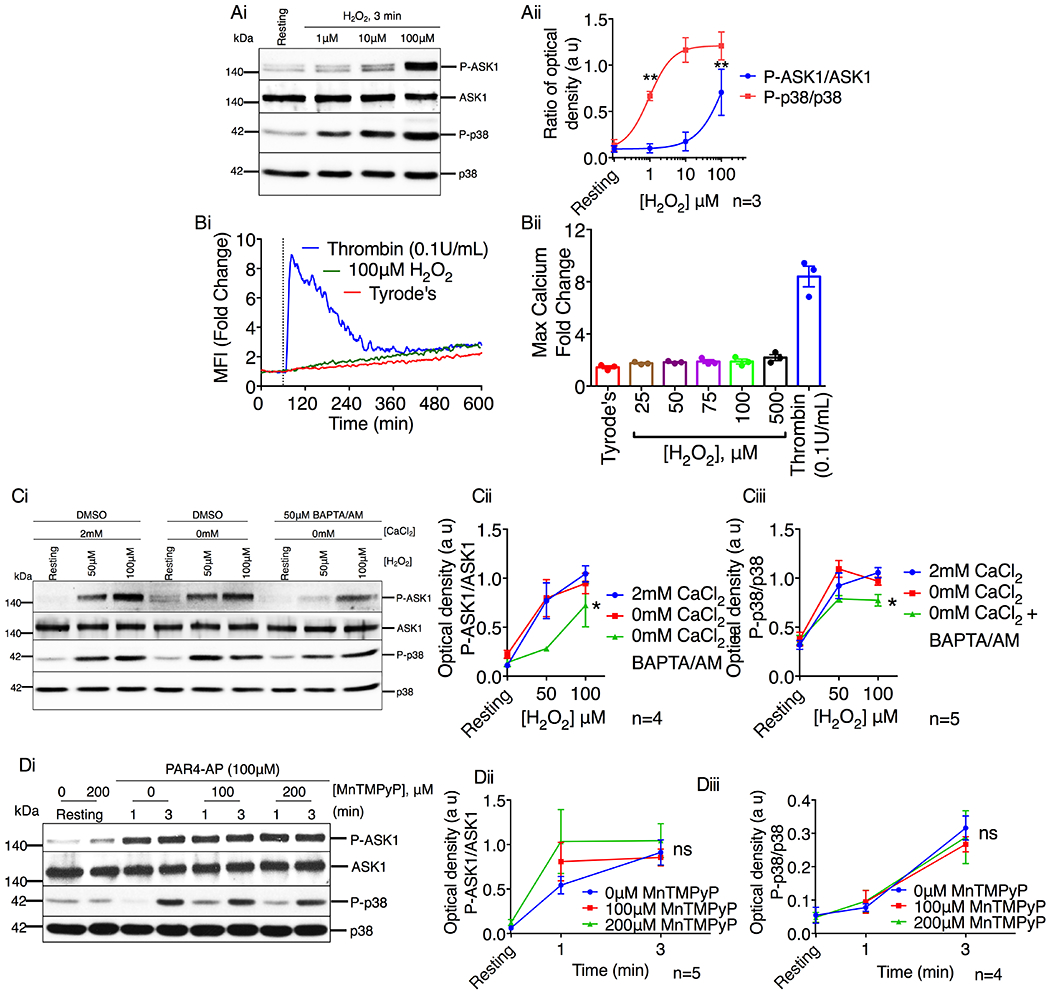
(A) Western blot analysis of human platelets stimulated with H2O2 (1–100μM). (Ai) Images of representative immunoblots of phosphorylation of ASK1 and p38 using phospho-specific antibodies. Blots were reprobed with anti-ASK1 and anti-p38 antibodies to ensure equal protein loading. (Aii) Quantification of the band intensities of P-ASK1 and P-p38 from (Ai). **P<0.01, one-way ANOVA with Dunnett’s multiple comparisons test. (B) Intracellular Ca2+ rise was assessed by flow cytometry in washed human platelets stimulated with thrombin or H2O2. (Bi) Representative tracing of thrombin (0.1U/mL) or H2O2 (100μM) induced intracellular Ca2+ rise in washed human platelets. (Bii) Quantification of the maximum mean fluorescence intensity (fold change). *P<0.05, one-way ANOVA with Dunnett’s multiple comparisons test. (C) Western blot analysis of human platelets in the presence or absence of extracellular Ca2+ and pretreated with either DMSO or BAPTA/AM (50μM) before being stimulated with H2O2 (50 & 100μM). (Ci) Images of representative immunoblots of phosphorylation of ASK1 and p38 using phospho-specific antibodies. Blots were reprobed with anti-ASK1 and anti-p38 antibodies to ensure equal protein loading. Quantification of the band intensities of (Cii) P-ASK1 and (Ciii) P-p38 from (Ci), *P<0.05, two-way ANOVA. (D) Western blot analysis of human platelets pretreated with vehicle or MnTMPyP (100 & 200μM) and stimulated with PAR4-AP (100μM). (Di) Images of representative immunoblots of phosphorylation of ASK1 and p38 using phospho-specific antibodies. Blots were reprobed with anti-ASK1 and anti-p38 antibodies to ensure equal protein loading. Quantification of the band intensities of (Dii) P-ASK1 and (Diii) P-p38 from (Di), Non significant (ns), Two-way ANOVA.
