Figure 9. ASK1 activation is negatively regulated by CIB1 in platelets.
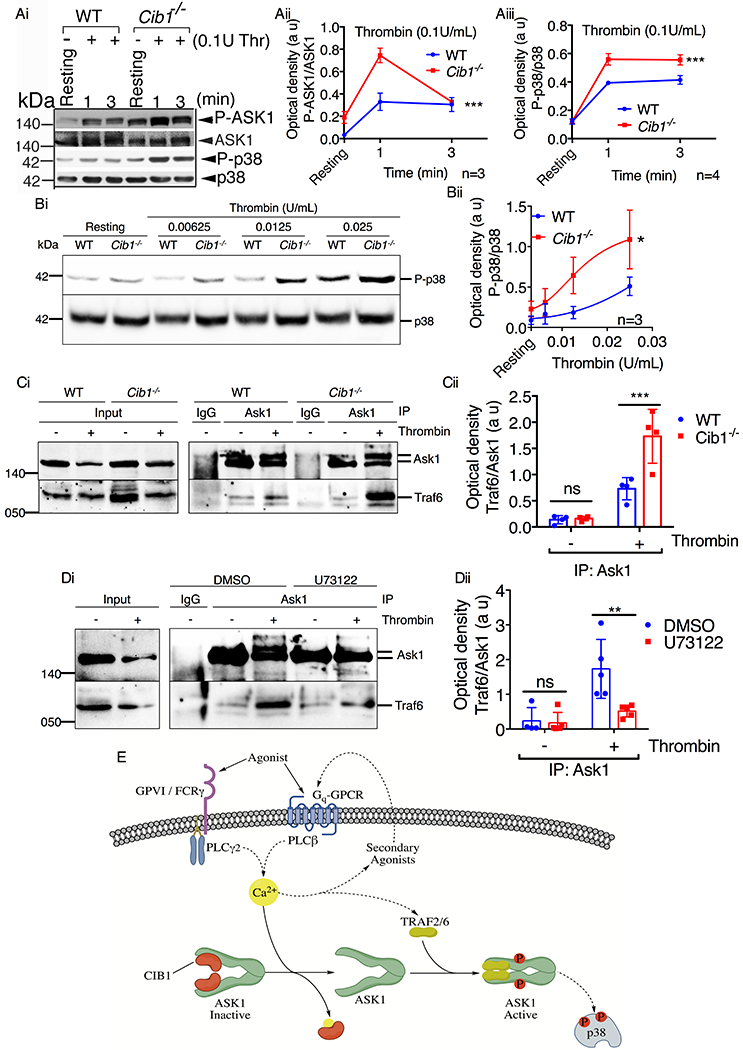
(A & B) Western blot analysis of washed murine platelets isolated from WT or Cib1−/− mice, pretreated with 200μM RGDS (5 min) and stimulated with either (A) thrombin (0.1U/mL) for 1 and 3 min or (B) thrombin (0.00625-0.025U/mL) for 3 min. Images of representative immunoblots of phosphorylation of (Ai) ASK1 and (Ai & Bi) p38 using phospho-specific antibodies. Blots were reprobed with (Ai) anti-ASK1 and (Ai & Bi) anti-p38 antibodies to ensure equal protein loading. Quantification of the band intensities of (Aii) P-ASK1 and (Aiii & Bii) P-p38 from (Ai & Bi) respectively, *P<0.05, ***P<0.001, two-way ANOVA. (C) Representative western blot images of immunoprecipitate of anti-Ask1 or normal rabbit IgG antibodies from resting and thrombin (1U/mL) stimulated platelet lysates and blotted for (Ci) Traf6. Blots were reprobed with anti-Ask1 antibody to ensure equal loading. (Cii) Quantification of the band intensity from (Ci); Non significant (ns), ***P<0.001, two-way ANOVA. (D) Representative western blot images of immunoprecipitate of anti-Ask1 or normal rabbit IgG antibodies from resting and thrombin (1U/mL) stimulated platelet lysates pretreated with either DMSO or U73122 (10μM) and blotted for (Di) Traf6. Blots were reprobed with anti-Ask1 antibody to ensure equal loading. (Dii) Quantification of the band intensity from (Di); Non significant (ns), **P<0.01, two-way ANOVA. (E) Schematic representation of the mechanism of agonist-induced ASK1 activation in platelets. In resting platelets (low Ca2+ levels) CIB1 is bound to ASK1 keeping it inactive. Platelet agonists, via their respective receptors, initiate PLCβ/γ activation causing an increase in intracellular Ca2+ concentration. Increased Ca2+ binds ASK1-bound CIB1 and dissociates it from ASK1. Free of its negative regulatory element, ASK1 then enters an inactive, intermediary state. From this intermediary state, ASK1 is then able to bind its positive regulatory elements TRAF2/6 to facilitate N-terminal dimerization and activation of ASK1 in platelets.
