Fig. 6.
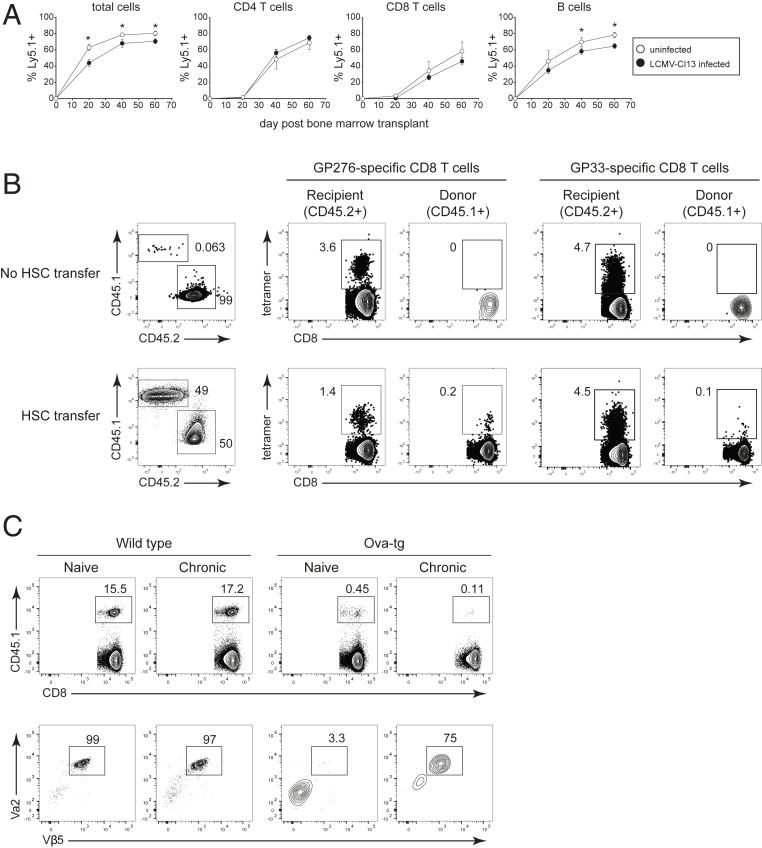
HSC-mediated generation of new LCMV-specific CD8 T cells is accompanied by escape of self-reactive T cells. All mice were CD4 depleted and then either left uninfected or infected with LCMV-Cl13 (generating a life-long viremic infection). All mice then received busulfan and transplant of CD45.1+ HSCs on day 30 after LCMV-Cl13 infection (or after 30 d in the case of the uninfected mice). (A) Donor (CD45.1+) lymphocytes in the peripheral blood of uninfected mice (white circles) or chronically infected mice (black circles) on the indicated day after HSC transplant. (B, Left) Flow plots show the frequency of recipient (CD45.2) and donor (CD45.1) CD8 T cells in the spleen 12 wk after the busulfan treatment and CD45.1+ HSC transplant. In this experiment, some mice received busulfan but not HSC transplant. Flow plots on the right show the frequency of LCMV-GP276–286 and LCMV-GP33–41 tetramer staining CD8+ T cells (gated on recipient or donor CD8 T cells). Note, “cells” in the CD45.1 gate in the No HSC transfer group represent background staining from the antibody and serve as a control for the lack of background tetramer staining. (C) Naïve or LCMV- Cl13 infected WT and Ova-transgenic (tg) mice were treated with busulfan and received transplant of 80% WT (CD45.2+) HSC plus 20% OT-1 (CD45.1) HSC. Both naïve mice and mice that were subsequently infected with LCMV-Cl13 were CD4 depleted in parallel at the start of the experiment. (Top) Flow plots show the frequency of OT-1 HSC-derived CD45.1+ cells in the spleen. (Bottom) Flow plots show TCR Vα2 and Vβ5 staining of the CD45.1+ (OT-1 HSC) donor cells in the spleen. Data are representative of two independent experiments with three to five mice per group. Error bars indicate SD. *P < 0.05.