1. Characteristics of patients in training and validation cohorts.
| Characteristics | Training cohort [n (%)] | P | Validation cohort [n (%)] | P | ||
| HER2− | HER2+ | HER2− | HER2+ | |||
| CEA, carcinoembryonic antigen; CT, computed tomography; HER2, human epidermal growth factor receptor 2; HER2−, HER2-negative patients; HER2+, HER2-positive patients; Rad-score, radiomics score. P value was calculated with the univariable association analysis between each of the clinical variables and HER2 status. | ||||||
Age (
 ) (year)
) (year)
|
64.61±8.39 | 63.38±8.25 | 0.497 | 59.20±8.87 | 64.60±10.03 | 0.084 |
| Sex | 0.336 | 0.379 | ||||
| Male | 47 (75.8) | 27 (84.4) | 21 (84.0) | 10 (66.7) | ||
| Female | 15 (24.2) | 5 (15.6) | 4 (16.0) | 5 (33.3) | ||
| CEA level | 0.002 | 0.174 | ||||
| Normal | 54 (87.1) | 19 (59.4) | 23 (92.0) | 11 (73.3) | ||
| Abnormal | 8 (12.9) | 13 (40.6) | 2 (8.0) | 4 (26.7) | ||
| Tumor location | 0.937 | 0.027 | ||||
| Upper-third | 19 (30.6) | 10 (31.2) | 5 (20.0) | 8 (53.3) | ||
| Middle-third | 10 (16.1) | 6 (18.8) | 7 (28.0) | 0 (0) | ||
| Lower-third | 33 (53.2) | 16 (50.0) | 13 (52.0) | 7 (46.7) | ||
| Clinical stage | 0.109 | 0.229 | ||||
| I | 18 (29.0) | 5 (15.6) | 5 (20.0) | 6 (40.0) | ||
| II | 19 (30.6) | 6 (18.8) | 7 (28.0) | 1 (6.7) | ||
| III | 24 (38.7) | 20 (62.5) | 12 (48.0) | 8 (53.3) | ||
| IV | 1 (1.6) | 1 (3.1) | 1 (4.0) | 0 (0) | ||
| CT-reported T stage | 0.140 | 0.476 | ||||
| T1−2 | 25 (40.3) | 8 (25.0) | 6 (24.0) | 6 (40.0) | ||
| T3−4 | 37 (59.7) | 24 (75.0) | 19 (76.0) | 9 (60.0) | ||
Rad-score (
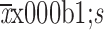 )
)
|
−0.761±0.235 | −0.501±0.248 | <0.001 | −0.762±0.227 | −0.534±0.316 | 0.023 |
