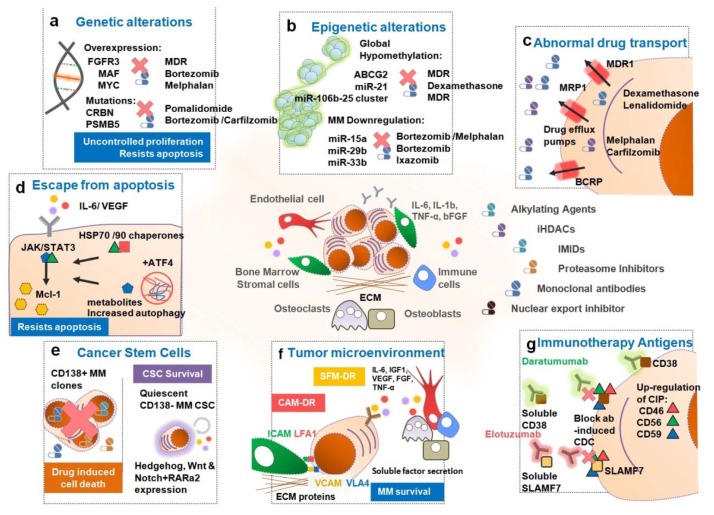
Figure 3.
Mechanisms of Drug Resistance enforced by MM. (a) Genetic alterations such as t(4;14), t(14;16) and t(14;20) translocations, 17p and 13p deletions and c-Myc associated abnormalities are associated with an unfavorable prognosis and insufficient response to current treatments. Additionally, (b) epigenetic alterations induced by a global hypomethylation of the DNA leads to the abnormal expression of several genes such as ATP binding cassette super-family G member 2 (ABCG2) and several miRs (e.g., miR-21, -15a, -29b, etc.) in malignant plasma cells (PCs) conferring a multidrug resistance (MDR) phenotype. (c) The overexpression of drug efflux pumps, namely P-glycoprotein (P-gp), in the malignant PCs mediates the cellular efflux of several drugs lowering intracellular drug concentration to sub-lethal levels. Moreover, (d) alterations in NF-κB, phosphatidylinositol 3-kinase/ protein kinase B (PI3K/AKT), mitogen-activated protein kinase/ extracellular signal-regulated kinase (MAPK/ERK) and Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3) signaling pathways in these PCs confers resistance to apoptotic stimuli and evasion to drug-induced cell death. (e) CD138- MM putative cancer stem cells are intrinsically resistant to most drugs. This small subset of cancer stem cells (CSCs) will survive therapy and remain as undetected/quiescent residual disease. Later on, these CSCs have the ability to self-initiate MM and cause refractory post-treatment relapse. (f) The bone marrow microenvironment is essential for MM survival, development and drug resistance by secretion of soluble factors e.g., interleukin 6 (IL-6), insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), B-cell activating factor (BAFF), fibroblast growth factor (FGF), stromal cell-derived factor 1α (SDF1α), and tumor necrosis factor-α (TNF-α). (g) Immunotherapy antigens: the anti-CD38 and SLAM7 monoclonal antibodies (mAbs), daratumumab and elotuzumab, fail to reach therapeutic efficacy either due to extracellular binding of the mAbs to target antigens or to upregulation of cell surface expression of the complement-inhibitors proteins CD46, CD56 and CD59.