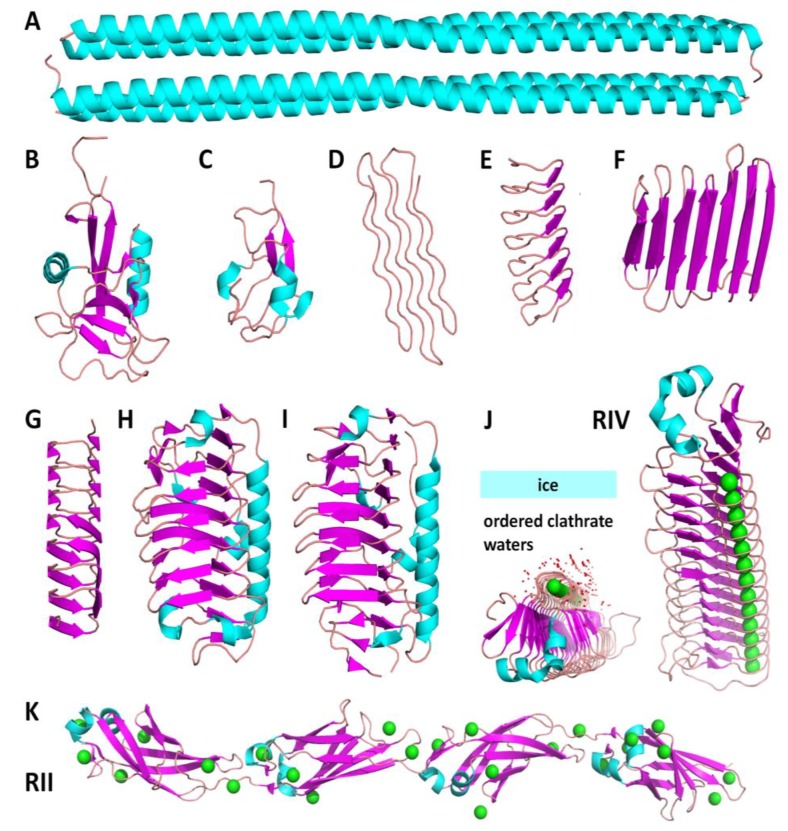
Figure 2.
Structures of the antifreeze proteins (AFPs) of different origins. Models of the experimental structures were accessed from the Protein Data Bank (PDB) and presented as a cartoon view. Cyan indicates an α-helix, magenta—a β-strand, pink—a loop, red—waters, green—Ca2+ ions. (A–C) fish AFPs (A—hyper active type I AFP from winter flounder, PDB: 4KE2; B—type II AFP from longsnout poacher, PDB: 2ZIB; C—type III AFP from European eelpout, PDB: 4UR4). (D–F) insect AFPs (D—snow flea AFP, PDB: 2PNE; E—AFP from Tenebrio molitor beetle, PDB: 1EZG; F—AFP from Rhagium inquisitor, PDB: 4DT5). (G) Plant AFP from perennial ryegrass, PDB: 3ULT. (H–J) microbial AFPs (H—AFP from an Antarctic sea ice bacterium Colwellia, PDB: 3WP9; I—AFP from Arctic yeast Leucosporidium, PDB: 3UYV; J–K—MpAFP from Marinomonas primoryensis: RIV—ice binding with Ca2+ ions in a regular arrangement and ordered waters, PDB: 3P4G, and linker RII, PDB: 4P99).