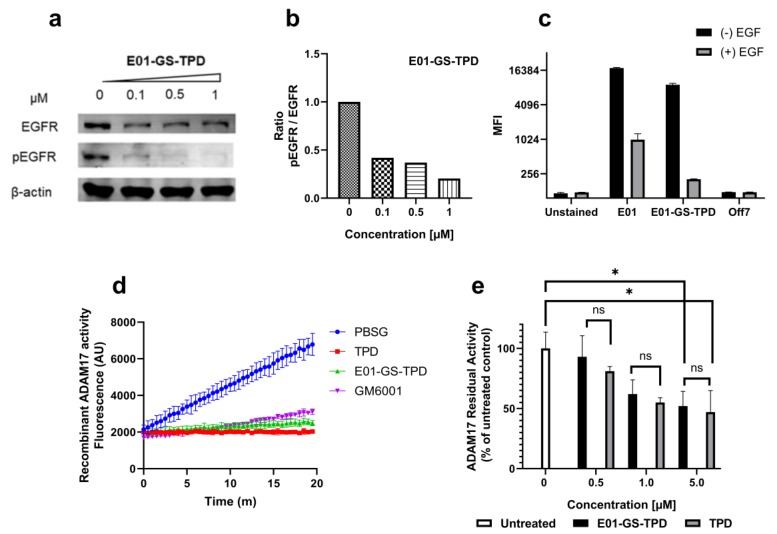
Figure 3.
E01-GS-TPD inhibits EGFR phosphorylation and ADAM17 activity in vitro. (a) A431 cells were treated with fusion protein E01-GS-TPD at increasing concentrations for a total of 48 h; the levels of total EGFR and phosphorylated EGFR (pTyr1068) were analyzed by Western blot. (b) the levels of phosphorylated EGFR relative to total EGFR were calculated based on band intensity and normalized to the untreated control (0 µM). (c) to evaluate ligand competition for receptor binding, A431 cells were treated with E01-sfGFP or E01-GS-TPD-sfGFP at 1 µM in the presence or absence of EGF (2 µM). Mean fluorescence intensity was measured. (d) to examine the ability of E01-GS-TPD to inhibit ADAM17 activity, recombinant ADAM17 was co-incubated with TPD, E01-GS-TPD, or the broad-spectrum matrix metalloproteinase (MMP) inhibitor GM6001 (2 µM). The increase in fluorescence was monitored, demonstrating inhibition compared to buffer-treated samples. (e) additionally, an assay measuring residual activity in live cells using a tumor necrosis factor (TNF)α-based fluorogenic substrate was performed as previously described [20]. E01-GS-TPD displayed a similar ADAM17 inhibitory profile compared to the TPD monomer at the tested concentrations (mean and standard deviation from n = 3 where possible is shown, *p < 0.05 in Tukey’s multiple comparisons test).