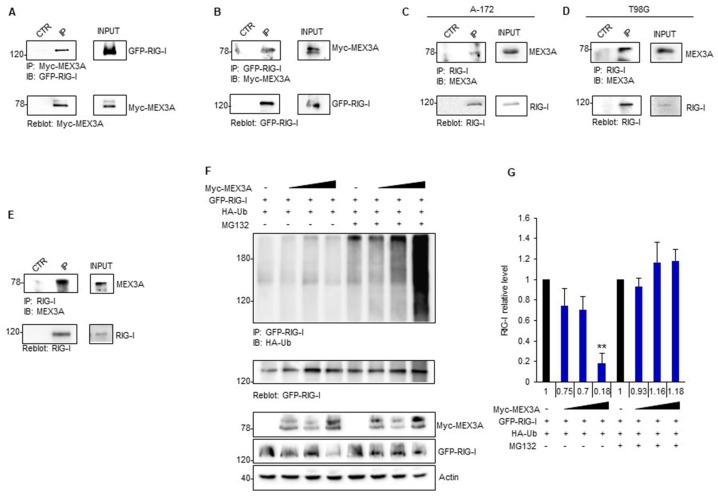
Figure 5.
MEX3A interacts with RIG-I mediating its ubiquitylation. (A,B) HEK293T cells were co-transfected with GFP-RIG-I and Myc-MEX3A in a (1:0.5) ratio, respectively. Interaction between RIG-I and MEX3A was detected by immunoprecipitation, followed by immunoblot analysis with the indicated antibodies. (C,D) Interaction between endogenous RIG-I and MEX3A was assessed by immunoprecipitation and immunoblotting in A-172 (C) and T98G (D) GB cell lines. (E) Binding between endogenous RIG-I and MEX3A was assessed by co-immunoprecipitation assay in GB clinical sample. (F) GFP-RIG-I was immunoprecipitated from HEK293T expressing the indicated proteins and treated with MG132 (50 μM) or control for 4 h, followed by immunoblotting with an anti-HA antibody to detect conjugated HA-Ub. Ubiquitylation blot was re-probed with a GFP antibody, to detect the immunoprecipitated level of RIG-I. Bottom GFP-RIG-I and Myc-MEX3A protein levels in total cell lysate. (G) Densitometric analysis of the levels of the indicated protein shown in (F) represents the mean of three independent experiments. Mean ± SD; ** p < 0.01 calculated using two-tailed Student’s t-test.