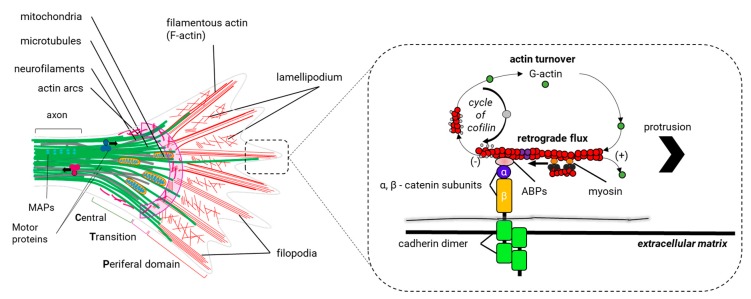
Figure 10.
Molecular structure of the cytoskeleton in the growth cone. The growth cone can vary its shape and size very fast, but usually, three domains are distinguished: the central (C) and peripheral (P) domains, and an intermediate zone called the transition zone (T) (left). The growth cone is an expanded and motile structure where several regions can be distinguished. In the axonal axis, microtubules (MTs) (green lines) are organized in parallel bundles by the microtubule-binding proteins (MAPs), such as tau (cyan spheres), whereas in the C domain, MTs are dispersed and are extended individually through the T zone until they reach the P domain. Here, MTs are aligned with the bundles of actin filaments (F-actin, red lines) in the filopodium. Here, actin filaments are organized in parallel beams that are extended back through the P domain to end up in the T zone, where they are cut in short filaments by a still unknown mechanism. F-actin in the lamellipodium is organized in branched networks. Actin arcs (fuchsia lines), composed by anti-parallel beams of F-actin and the motor protein myosin II (not shown in the graph) are located in the T zone and at the periphery of the C domain. Actin arcs produce the compressive forces in the C domain that bend the scattered MTs and facilitate their grouping in the wrist of the growth cone. Motor proteins supply the growth cone with the materials and organelles necessary for the formation of the new axonal segment (anterograde transport) and they transport vesicles involved in the endocytic pathways (retrograde transport). In the filopodia (right), the balance between polymerization and the retrograde flow of F-actin controls the protrusion of the growth cone. F-actin is disassembled in the T zone and is polymerized in the filopodia of the growth cone. The protrusion phase occurs when the polymerization rate of F-actin exceeds that of the retrograde flow. Once the filopodium recognizes an adhesive substrate in the extracellular matrix, it binds to it using adhesion proteins such as cadherins, which in turn interact with heterodimeric transmembrane receptors of α/β—integrin in the growth cone. These receptors interact with some actin-binding proteins (e.g., vinculin, talin, paxillin) to communicate F-actin with the extracellular matrix. Src protein kinase and the focal adhesion kinase (FAK) bind to actin-binding proteins (ABPs) to activate the adhesion of other additional proteins (not shown in this figure). It is believed that the union of the ABPs restricts the retrograde flow and strengthens the polymerization of actin to generate the protrusion of the membrane.