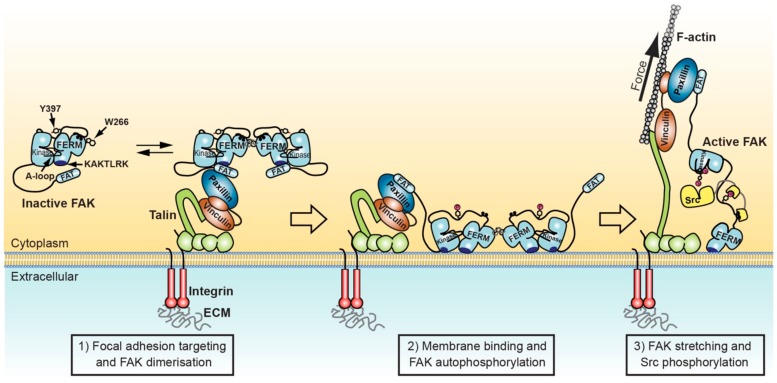
Figure 2.
Model for FAK activation in focal adhesions mediated by membrane interactions and force. Left: FAK can form dimers via F3 lobe interactions involving W266. FAK dimer formation is likely promoted upon recruitment into focal adhesions by increased local concentration and potentially by paxillin-FAT interactions that synergise with FAT-FERM interactions to stabilise FAK dimers. Middle: FAK interacts with nearby PIP2 rich membranes via the KAKTLRK basic patch in the FERM F2 lobe. Increased avidity for PIP2 of FAK dimers might enhance membrane attachment. Membrane interactions induce conformational changes that allow simultaneous interactions of FERM and kinase domains to the membrane and expose the FERM-kinase linker for efficient autophosphorylation on Y397. Right: Src binds with its SH2 domain to the phosphorylated Y397 site and with the SH3 domain to the PxxP motif in the FERM-kinase linker. Stretching forces applied to FAK by contracting actomyosin fibres cause release of the FAK kinase domain from FERM and membrane interactions, exposing the activation loop for efficient phosphorylation by Src, resulting in FAK activation.