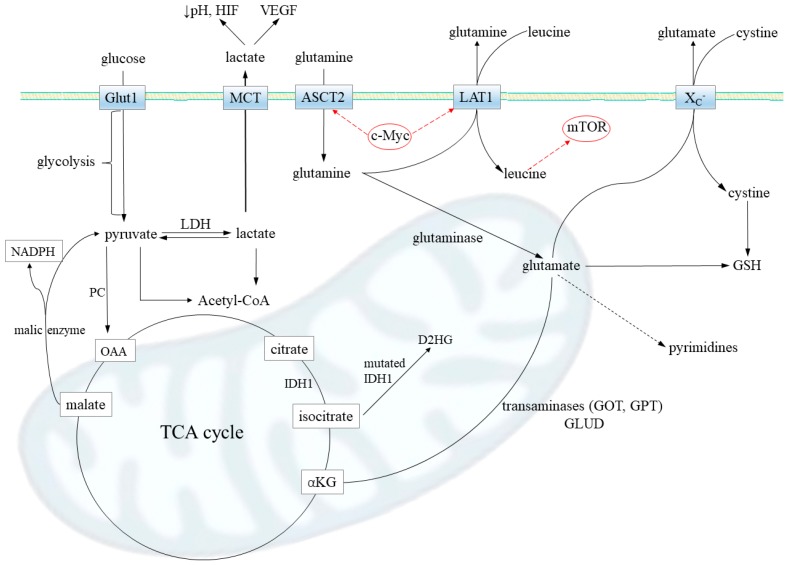
Figure 2.
Metabolic pathways contributing to glutamine addiction in gliomas. Firstly, glutamine transport via Slc1a5 transporter (ASCT2) is increased in gliomas. The promoters of the Slc1a5 gene are under transcriptional control of oncogenic transcription factor c-MYC, which upregulates its expression. A substantial portion of intracellular glutamine is released from the cell via transporter LAT1 (Slc7a5; also upregulated by c-MYC) in exchange for extracellular leucine, an essential amino acid. The glutamine–leucine shuttle directly activates mTOR signaling, leading to increased protein synthesis. That way, an abundance of glutamine further accelerates the glioma anabolism. When in the cell, glutamine may be converted by glutaminase to glutamate, which is then metabolized to (i) alpha-ketoglutarate (αKG) (by transaminases GPT and GOT or glutamate dehydrogenase, GLUD) and serves for tricarboxylic acid (TCA) cycle anaplerosis; the reaction catalyzed by pyruvate carboxylase (PC), generating oxaloacetate (OAA) from pyruvate, is downregulated in most neoplasms; (ii) oncometabolite D-2-hydroxyglutate (D2HG) in the case of mutations in IDH1 enzyme, leading to a loss of proper enzymatic activity; iii) antioxidant glutathione (GSH), responsible for the treatment resistance; transporter XC- performs antiport of glutamate and cystine, which is a limiting substrate in GSH synthesis; and iv) lactate, which may be a source of energy when glutaminolysis takes place and the malate shuttle operates (malic enzyme synthetize lactate precursor, pyruvate, and NADPH) but also modulate tumor invasiveness via vascular endothelial growth factor (VEGF) and hypoxia-inducing factor (HIF); lactate was shown to stabilize HIF and then transactivate c-Myc in a pathway that mimics a response to hypoxia. In concordance to high lactate release observed in glioma, the lactate dehydrogenase (LDH), responsible for lactate synthesis, has been often reported to be upregulated. Last, but not least, glutamine availability is a limiting step for pyrimidine synthesis that highly proliferative cells, such as gliomas, need to be kept high.