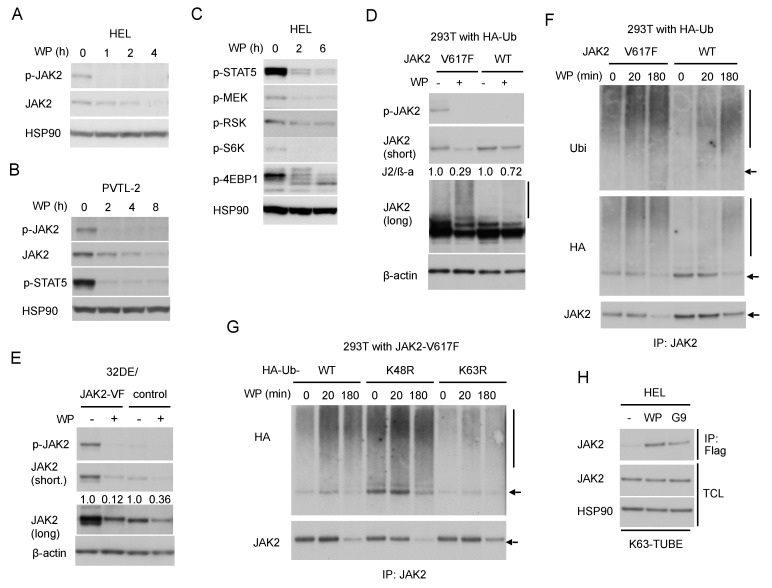
Figure 2.
WP1130 enhances K63-linked polyubiquitination and preferentially downregulates the phosphorylated form of JAK2-V617F to inhibit downstream signaling. (A,B,C) HEL (A,C) or PVTL-2 (B) were treated with 5 µM WP1130 (WP) for indicated times. Cells were lysed and subjected to immunoblot analysis with antibodies against indicated proteins. HSP90 was used for a loading control. (D) 293T cells transfected with plasmids coding for either JAK2-V617F (V617F) or wild-type JAK2 (WT) and HA-tagged ubiquitin were treated for 3 h with or without 5 µM WP1130, as indicated, and analyzed. β-actin was used for a loading control. Short and long exposure results are shown where indicated. Relative expression levels of JAK2 as compared with those in untreated cells analyzed by densitometry and normalized by that of ß-actin (J2/β -a) are indicated. The vertical line indicates the smeary pattern characteristic of polyubiquitination. (E) 32DE/JAK2-V617F cells or vector control cells cultured with Epo were treated with or without 5 µM WP1130 for 2 h, as indicated, and analyzed. (F) 293T cells transduced with HA-tagged ubiquitin along with either JAK2-V617F or wild-type JAK2 were treated for indicated times with 5 µM WP1130 and lysed. Immunoprecipitates (IP) obtained with anti-JAK2 were analyzed. The arrow indicates the position corresponding to JAK2. (G) 293T cells transduced with JAK2-V617F along with HA-tagged ubiquitin (WT) or its K48R or K63R mutant were treated with 5 µM WP1130 for indicated times and analyzed. (H) HEL cells were left untreated as control or treated for 30 min with 5 µM WP1130 or 3 µM G9 as indicated. K63-polyubiquitinated proteins were isolated by immunoprecipitation with anti-Flag after incubation of cell lysates with FLAG-K63-TUBE (K63-TUBE). Immunoprecipitates, as well as total cell lysates (TCL), were analyzed.