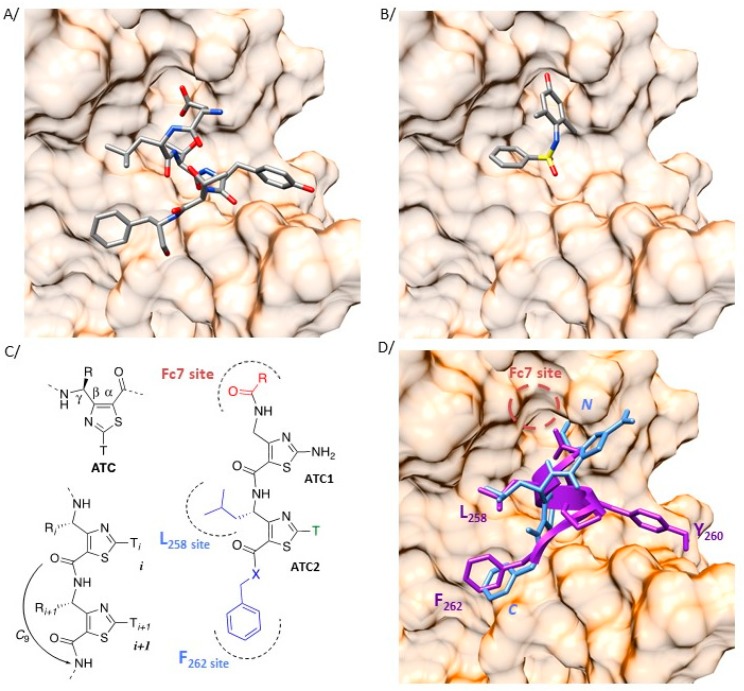
Figure 2.
Heteroaromatic γ-dipeptide to mimic the auto inhibitory domain of cytohesin proteins. (A) Crystal structure (2R09) of the region of the guanine nucleotide exchange factor general receptor of phosphoinositides-1 (GEF GRP1) interacting with the switch I and switch II regions of Arf proteins (surface representation). The intrinsic autoinhibitory peptide of GRP1 (257-DLTYTF-262) is represented in stick (stick representation, colored by elements with carbon in grey, oxygen in red, nitrogen in blue, and sulfur in yellow). (B) Crystallographic pose (4JWL) of Fc7 (stick representation, colored by elements, as previously described) at the same region of the Sec7 domain of Arno (surface representation). (C) Nomenclature of 4-amino-(methyl)-1,3-thiazole-5-carboxylic acid (ATC) γ-amino acids and characteristic H-bonding network of the oligomers. In the designed ATC dipeptides, the substituents in blue point towards the L258 and F262 binding sites while the Fc7 binding site is targeted by the N-ter extremity (red). (D) The intrinsic autoinhibitory peptide of GRP1 (257-DLTYTF-262) and the γ-dipeptide based mimic are represented in purple ribbon and blue stick, respectively. The three-dimensional (3D) side chain projection around the ATC scaffold was determined from the ATC XRD structure (CSD entry AGEZAP/922304). The Fc7 binding site is indicated as a red dash circle.