Abstract
Chronic kidney disease (CKD) is one of the fastest growing causes of death worldwide, emphasizing the need to develop novel therapeutic approaches. CKD predisposes to acute kidney injury (AKI) and AKI favors CKD progression. Mitochondrial derangements are common features of both AKI and CKD and mitochondria-targeting therapies are under study as nephroprotective agents. PGC-1α is a master regulator of mitochondrial biogenesis and an attractive therapeutic target. Low PGC-1α levels and decreased transcription of its gene targets have been observed in both preclinical AKI (nephrotoxic, endotoxemia, and ischemia-reperfusion) and in experimental and human CKD, most notably diabetic nephropathy. In mice, PGC-1α deficiency was associated with subclinical CKD and predisposition to AKI while PGC-1α overexpression in tubular cells protected from AKI of diverse causes. Several therapeutic strategies may increase kidney PGC-1α activity and have been successfully tested in animal models. These include AMP-activated protein kinase (AMPK) activators, phosphodiesterase (PDE) inhibitors, and anti-TWEAK antibodies. In conclusion, low PGC-1α activity appears to be a common feature of AKI and CKD and recent characterization of nephroprotective approaches that increase PGC-1α activity may pave the way for nephroprotective strategies potentially effective in both AKI and CKD.
Keywords: kidney, mitochondrial biogenesis, sirtuin, PGC-1α, oxidative stress, diabetes, acute kidney injury
1. AKI, CKD and the Mitochondrial Connection
Kidney diseases represent a growing worldwide burden and may cause acute kidney injury (AKI) and/or chronic kidney disease (CKD). AKI is characterized by a rapid, often reversible loss of renal function. Current AKI mortality remains around 50% [1]. There is no effective treatment for AKI that accelerates recovery. The only treatment is symptomatic and consists of replacement of renal function by dialysis in severe cases [2]. Key causes of AKI include sepsis, ischemia-reperfusion injury (IRI) and nephrotoxic agents [2,3].
The first events during AKI, sublethal cell injury, cell death, and inflammation, are followed by regeneration leading to kidney function recovery, but suboptimal regeneration may result in transition to CKD [4]. The initial wave of tubular cell death is followed by tubular cell proliferation favoring regeneration, and by a second wave of cell death that adjusts the final cell number [5,6]. Regulated necrosis cell death through ferroptosis and necroptosis releases inflammatory cytokines that amplify tissue injury [7,8] while there is also evidence for a contribution of apoptotic cell death to kidney injury [9].
CKD is among the fastest growing global causes of death; it is projected to become the fifth global cause of death by 2040 and the second cause of death in long-lived countries before the end of the century [10,11]. CKD is usually irreversible and is defined by decreased kidney function assessed by glomerular filtration rate (GFR, <60 mL/min per 1.73 m2), or evidence of kidney injury, such as pathological albuminuria (>30 mg/g of urinary creatinine) persisting for at least 3 months [12,13]. End-stage kidney disease requires kidney function replacement by dialysis or kidney transplantation [14]. Therapeutic options to prevent CKD progression are limited, contributing to the high burden of disease. Thus, it is imperative to develop new therapeutic options for AKI and CKD. Research approaches may take advantage of the fact that AKI and CKD are interconnected syndromes to target common pathogenic pathways [15,16,17,18]. Thus, AKI episodes favor CKD progression, and CKD is a risk factor of AKI. A key role of mitochondria in kidney injury has been suggested by interventional studies [19]. In fact, mitochondrial dysfunction is a key factor in the pathogenesis of AKI, causing tubular cell dysfunction and death [20,21]. Mitochondrial homeostasis results from fusion or fission of pre-existing mitochondria, degradation of dysfunctional mitochondria via mitophagy or the ubiquitin proteasome system, and generation of new mitochondria from existing mitochondria via mitochondrial biogenesis [22,23]. We now review the contribution to kidney disease of the altered expression and activity of a key transcription factor in mitochondrial biogenesis: Peroxisome proliferator-activated receptor-γ coactivator-1-α (PGC-1α). Specifically, after an overview of the function of PGC-1α in mitochondrial biogenesis, its role in health and disease, and the involvement of mitochondria in kidney disease, we review evidence supporting the concept that PGC-1α derangements contribute to kidney disease and can be therapeutically targeted.
2. PGC-1α: A Regulator of Mitochondrial Biogenesis
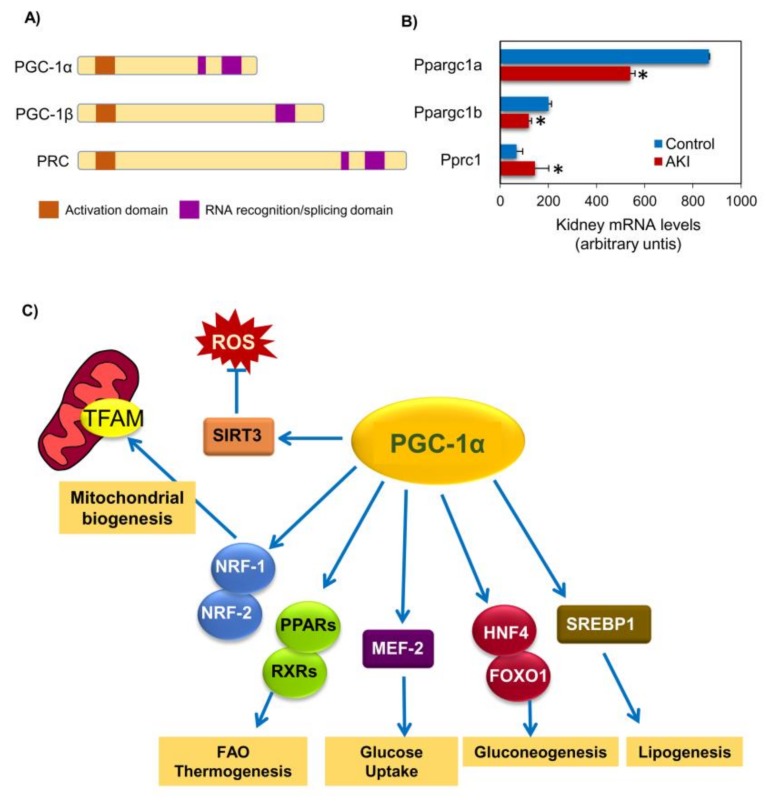
Mitochondrial biogenesis is a complex process that involves synthesis of the inner and outer mitochondrial membranes and mitochondrial encoded proteins synthesis and import of nuclear encoded mitochondrial proteins and replication of mitochondrial DNA (mtDNA), requiring a coordinated regulation of two distinct genomes, the nuclear and mitochondrial genomes, given that a majority of mitochondrial proteins are encoded by nuclear genes [22,23]. PGC-1α is a master regulator of mitochondrial biogenesis that coordinates the transcriptional machinery leading to increased mitochondrial mass thus allowing tissue adaptation to increased energetic demands [23]. PGC-1α is encoded by the PPARGC1A gene and belongs to the PGC-1 family, also composed of PGC-1β (encoded by PPARGC1B), which contributes to maintain basal mitochondrial function, and PRC (PGC-1-related coactivator, encoded by PPRC1), which appears to be restricted to regulating mitochondrial biogenesis in proliferating cells [24] (Figure 1A). While there is abundant literature on PGC-1α and kidney disease, as discussed below, much less is known about the role of PGC-1β and PRC in kidney disease, despite the fact that transcriptomic studies have identified them as differentially expressed during experimental AKI [25,26,27] (Figure 1B).
Figure 1.
Peroxisome proliferator-activated receptor-γ coactivator-1-α (PGC-1α) structure, family and functions. (A) Structure of the PGC-1α family of transcriptional regulators. (B) Gene expression of the PGC-1α family of transcriptional regulators during experimental folic acid-induced acute kidney injury (AKI) [28]. * False discovery rate <0.02 vs control. (C) Key PGC-1α functions. Although in the context of kidney disease and this review we will focus on the role of PGC-1α in mitochondrial biogenesis, it has multiple additional functions in metabolism homeostasis that involve different binding partners regulating gene transcription as well as multiple target genes and proteins. Abbreviations: FAO: fatty acid β-oxidation, FOXO1: fork head box O1, HNF-4: hepatic nuclear factor-4, MEF-2: myocyte enhancer factor-2, NRF-1/2: nuclear respiratory factor 1/2, PPARs: peroxisome proliferator-activated receptors, PRC: PGC-1-related coactivator, ROS: reactive oxygen species, RXR: retinoid receptors, Sirt3: sirtuin 3, STREBP1: sterol regulatory element-binding protein 1, TFAM: mitochondrial transcription factor A.
PGC-1α activity is regulated by both posttranslational modifications and by gene expression levels (Figure 2). PGC-1α can be activated by posttranslational modifications (e.g., phosphorylation or de-acetylation) or by increased transcription. Stressors (glucose deprivation, starvation or exercise) lead to AMP-activated protein kinase (AMPK)-mediated phosphorylation of PGC-1α as well as to increased nicotinamide adenine dinucleotide (NAD) levels resulting in activation of Sirtuin-1 (SIRT1), a NAD-dependent deacetylase which deacetylates PGC-1α. In preclinical studies, increased NAD biosynthesis reduces renal injury, but the mechanism is unclear given the multiple functions of NAD [29,30,31]. Active PGC-1α translocates into the nucleus where it activates nuclear respiratory factor 1 and 2 (Nrf1 and Nrf2), and subsequently the transcription of nuclear coded respiratory chain components and mitochondrial transcription factor A (Tfam) thus promoting synthesis of mitochondrial proteins, mtDNA replication and transcription, and new mitochondria biogenesis [22,23] (Figure 1C). PGC-1α may also interact with additional nuclear factors, thus regulating multiple pathways involved in cellular energy metabolism within and outside mitochondria. The list of partners includes PPARα, PPARβ, retinoid receptors (RXR), myocyte enhancer factor-2 (MEF-2), fork head box O1 (FOXO1), hepatic nuclear factor-4 (HNF-4), sterol regulatory element-binding protein 1 (SREBP1) or estrogen-related receptors (ERRs), among others (Figure 1C). These transcription factors regulate genes involved in mitochondrial fatty acid oxidation, lipogenesis, thermogenesis, and glucose metabolism [32], which are critical for tissues with high metabolic demands, such as heart, skeletal muscle, brain and kidney [22].
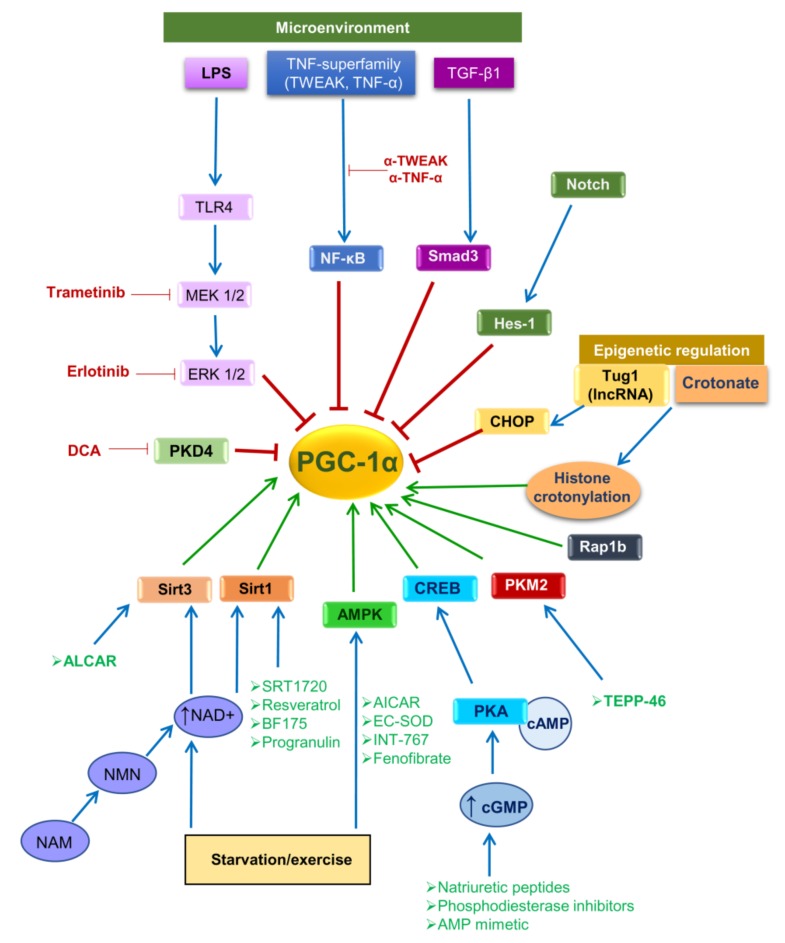
Figure 2.
Regulators of PGC-1α gene expression, activity and potential for therapeutic intervention aimed at increasing PGC-1α activity. The microenvironment (including mediators of inflammation and fibrosis), starvation/exercise and epigenetic regulation are the main modulators of PGC-1α activity. These, in turn, modulate intracellular signaling pathways that negatively (red lines) or positively (green lines) regulate PGC-1α gene expression and activity. Therapeutic approaches potentially upregulating PGC-1α gene expression and activity are depicted outside boxes: in green, those that activate specific intracellular signaling pathways to increase PGC-1α activity, and in red, those that inhibit specific intracellular signaling pathways to promote PGC-1α activity. Abbreviations: AICAR: 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside, ALCAR: antioxidant agent acetyl-l-carnitine, AMPK: AMP-activated protein kinase, cAMP: cyclic adenosine monophosphate, cGMP: cyclic guanosine monophosphate, CHOP: C/EBP homologous protein, CREB: cAMP responsive element binding protein, DCA: dichloroacetate, ERK: extracellular signal-regulated kinase, LPS: lipopolysaccharide, MEK: mitogen-activated protein kinase kinase, NAD: nicotinamide adenine dinucleotide, NAM: niacinamide, NF-κB: nuclear factor κB, NMN: nicotinamide mononucleotide, PDK4: pyruvate dehydrogenase kinase 4, PKA: protein kinase A, PKM2: pyruvate kinase M2, Rap1b: RAS-Related Protein Rap1b, Sirt1: sirtuin 1, Sirt3: sirtuin 3, TEPP-46: tetraethyl diphosphate 46, TGF-β1: transforming growth factor β1, TLR4: toll-like receptor 4, TNF-α: tumor necrosis factor α, Tug1: taurine up-regulated gene 1, TWEAK: TNF-like weak inducer of apoptosis.
PGC-1α mRNA levels also have positive and negative regulators (Figure 2). At the transcriptional level, cAMP response element-binding protein (CREB) activates PGC-1α transcription [22,33]. High cAMP or cGMP levels activate protein kinase A (PKA) which in turn, phosphorylates and activates CREB. cGMP is a key regulator of mitochondrial biogenesis and cGMP-specific phosphodiesterase (PDE) inhibitors, which increase cGMP by blocking its cleavage to GMP, promote PGC-1α expression and mitochondrial biogenesis in the heart and kidney [34,35]. Moreover, natriuretic peptides also stimulate cGMP synthesis and promote PGC-1α expression and mitochondrial biogenesis [36].
In contrast, inflammatory mediators such as TNF-α or TWEAK reduce PGC-1α expression and mitochondrial biogenesis through activation of nuclear factor-κB (NF-κB) and epigenetic regulation [37,38]. Furthermore, profibrotic factors may also reduce PGC1α expression. The profibrotic transcription factors Hes1, a downstream target of Notch signaling [39], can directly bind the PGC-1α promoter region and inhibit PGC-1α gene expression. Moreover, TGF-β1 decreases PGC-1α levels though epigenetic downregulation via Smad3 [40].
3. PGC-1α in Health and Disease
A role for PGC-1α has been described in diabetes and pancreatitis, liver disease, and endothelial cell injury, in addition to kidney disease.
3.1. Diabetes
PGC-1α is expressed in liver and pancreatic β cells, two key players in diabetes mellitus (DM). Liver PGC-1α is induced by glucagon and other mediators that increase intracellular cAMP levels and CREB activation [41]. In the liver, PGC-1α not only controls mitochondrial biogenesis and fatty acid oxidation, as in other organs but also drives fasting-induced gluconeogenesis [42]. Indeed, liver and pancreas PGC-1α expression is increased in diabetic patients and animals [43]. However, the role of PGC-1α in diabetes is still controversial, possibly due to the different functions of PGC-1α in different organs [44]. In pancreatic β-cells, forced PGC-1α expression inhibits glucose-stimulated insulin secretion in vitro and in vivo [45] and PGC-1α activity drives β-cell apoptosis in response to high glucose levels [46], suggesting that PGC-1α may favor DM development. The proposed mechanism was increased expression of uncoupling protein-2 (UCP-2), which is known to reduce glucose-stimulated insulin secretion [47,48]. UCP-2 is expressed under conditions driving mitochondrial dysfunction, including hyperglycemia, and reduces the mitochondrial membrane potential when the electron flow cannot be coupled to ATP synthesis. PGC-1α overexpression may also inhibit β-cell differentiation [49,50]. However, human genetic studies suggest a protective role of PGC-1α in DM [51]. Thus, Gly482Ser, the most common PPARGC1A polymorphism, is associated with Type 2 DM (T2DM), but results in decreased PGC-1α mRNA levels and insulin secretion [52] and insulin resistance [53]. In this regard, high glucose and palmitic acid (a key mediator of β-cell lipotoxicity) concentrations down-regulate PGC-1α levels [54,55] and inducible PGC-1α deletion in β-cells results in decreased insulin secretion [56]. These results suggest a general protective role of PGC-1α, that might be lost under disease conditions, and also, a tight regulation of the system in which excess inappropriate PGC-1α may be deleterious. The understanding of these relationships is key to developing PGC-1α-based therapeutic approaches for kidney disease since diabetic nephropathy which is the most frequent cause of CKD, and also predisposes to AKI [57]. In this regard, metabolomics identified a signature of mitochondrial dysfunction in human diabetic nephropathy, associated with lower PGC-1α gene expression and is evidence of an overall impaired mitochondrial biogenesis [58,59] (discussed below).
3.2. Pancreatitis
PGC-1α protects the pancreas from the complications of acute pancreatitis, which is more frequent and has poorer outcomes in obese subjects who have low pancreas PGC-1α levels. Thus, PGC-1α deficient mice were more sensitive to acute pancreatitis induced by cerulein due to a reduced capacity to control the resulting inflammatory response, leading to an uncontrolled over-activation of NF-κB and the subsequent induction of IL-6 [60].
3.3. Liver Disease
PGC-1α deficient mice are insulin sensitive and are not hypoglucemic in normal conditions but, when fasted, fail to induce gluconeogenesis and accumulate lipids in the liver, leading to liver steatosis [61]. Accordingly, PGC-1α levels are reduced in liver steatosis, a common condition that is a risk factor for liver disease and that yields transplanted livers more sensitive to IRI [62,63,64]. Loss of PGC-1α is a key factor in the enhanced susceptibility of steatotic livers to IRI and PGC-1α activity is necessary for ischemic preconditioning [65]. This effect is likely associated with the induction of antioxidant gene expression by PGC-1α. Similarly, PGC-1α protects from alcoholic and non-alcoholic fatty liver disease, from viral-induced steatohepatitis and from hepatotoxicity [66,67,68,69,70]. These protective effects may be related at least in part to the negative regulation of liver inflammation by PGC-1α. Importantly, in the damaged, inflamed liver, PGC-1α levels are further downregulated by inflammatory mediators like TNF-α [71].
Another liver-specific activity of PGC-1α is regulation of Selenoprotein P (SeP), which controls selenium homeostasis [72]. Selenium is a cofactor of selenoproteins that play key roles in cellular redox control [73]. In this regard, human livers express a liver-specific PGC-1α transcript (L-PGC-1α) resulting from using an alternative promoter [74]. While coactivation properties mostly overlap with the ubiquitous PGC-1α, there are functional differences. For example, L-PGC-1α seems unable to coactivate liver X receptor alpha (LXRα).
While traditionally the hepatorenal syndrome causing AKI was the main kidney-related concern in liver disease patients, more recently a link between liver steatosis, non-alcoholic fatty liver disease (NAFLD) and CKD has been emphasized [75,76]. Since NAFLD, diabetes and CKD are complications of the metabolic syndrome, this points to the potential utility of PGC-1α-based therapeutic approaches to target the different complications of metabolic syndrome.
3.4. Endothelium
Endothelial cells are generally regarded as glycolytic cells that make a very limited use of mitochondria. However, they do express PGC-1α that in these cells regulates antioxidant gene expression. Thus, PGC-1α prevented high glucose-induced endothelial dysfunction and increased eNOS expression and the synthesis of NO synthesis, a key modulator of vascular relaxation [77]. In fact, NO itself regulates endothelial PGC-1α expression [78]. PGC-1α may also protect from hyperlipidemia-induced endothelial dysfunction. Thus, C1q/TNF-Related Protein-9 protects from Oxidized-LDL-induced endothelial dysfunction via PGC-1α [79].
Loss of endothelial PGC-1α induced a mesenchymal transition characterized by reduced adhesion, increased migration, cytoskeletal disorganization and poor adherence junctions resulting in an exacerbated tip cell-like phenotype that responded poorly to angiogenic mediators, such as vascular endothelial growth factor A (VEGF-A) [80,81]. Tip cells at the end of developing blood vessels drive vessel formation. PGC-1α-deficient mice had a significant loss of pericyte coverage of retinal endothelial cells. Pericytes normally maintain vascular structures and pericyte loss correlates with reduced vascular perfusion [82]. In this regard, PGC-1α deficient mice developed more severe hyperoxia-induced retinal vascular abnormalities and retinopathy [82]. These results therefore suggest that PGC-1α may be protective in microvascular diseases, including retinopathies. In line with these results, PGC-1α heterozygous mice exposed to a high fat diet developed age-related macular degeneration-like abnormalities in the retinal pigmented epithelium and local inflammatory responses [83]. In the context of kidney diseases, endothelial dysfunction associated with hypertension, obesity and/or diabetes is a key mechanism in CKD progression and CKD-related cardiovascular complications [84,85,86,87]. Thus, prevention of endothelial injury may also be beneficial in the kidney disease context. In this regard, diabetic nephropathy is considered a manifestation of diabetic microvascular disease.
4. Mitochondria in Kidney Diseases
Due to the high demand for energy for solute reabsorption [88], the kidneys, and particularly proximal tubular and medullary thick ascending limb cells display a high mitochondrial density [89]. The main source of energy in the kidney is ATP generation from fatty acid β-oxidation (FAO) in tubular cell mitochondria, a process regulated by carnitine palmitoyl-transferase 1 (CPT1) as the limiting enzyme. Tubular cells are very sensitive to kidney insults, and mitochondria are key players in cell death, particularly in apoptotic cell death [90]. Additionally, mitochondria may get damaged and dysfunctional in the course of kidney injury. In this regard, there is increasing functional and interventional evidence supporting a key role for mitochondria in kidney disease.
4.1. Morphological and Functional Changes of Mitochondria
Kidney injury may alter mitochondrial structure and function in different manners, including alterations of calcium homeostasis, membrane integrity, ROS production, mitochondrial transport, biogenesis, dynamics (fusion/fission) and mitophagy [91,92,93]. Altered mitochondrial homeostasis may in turn further promote kidney injury triggering a harmful feedback loop [19,92,94,95,96].
Mitochondrial injury plays a key role in experimental AKI models triggered by sepsis [94,97,98], IRI [99,100,101,102,103], and nephrotoxicity [104,105,106]. Mitochondrial damage in AKI is associated with mitochondrial fragmentation [107], reduced mitochondrial mass [108], mitochondrial swelling and cristae disruption [109,110], apoptosis [111], and, in general, with impaired mitochondrial function [104,105,106]. Mutations or large deletions on mtDNA or nuclear genes encoding for mitochondrial proteins may result in kidney cysts or glomerular or tubular disease [112,113,114,115,116]. CKD and kidney tumors have also been linked to mitochondrial abnormalities. Dysmorphic mitochondria, reduced mtDNA [117] and decreased ATP production by mitochondria contribute to the development and progression of diabetic nephropathy [118,119] while chronic allograft nephropathy is characterized by reduced mitochondrial biogenesis, inadequate energy production and impaired antioxidant function [120]. In human CKD, lower renal expression of key FAO enzymes (such as CPT1 and 2), transcription factors (peroxisome proliferator-activated receptors) and other mitochondrial genes are associated with kidney fibrosis [121], suggesting a dysregulated cell metabolism in CKD. In this sense, transgenic and pharmacological approaches that restore FAO were beneficial in experimental kidney damage [121]. In CKD, tubular cells may undergo partial epithelial-to-mesenchymal transition and generate a senescence-like secretome that contributes to CKD progression. Senescence may be associated with mitochondrial injury and increased mitophagy [122,123]. Additionally, both defective DNA repair and early low renal mitochondrial activity are associated with benign renal tumors and renal cell carcinoma [124,125,126,127].
4.2. Mitochondria-Targeted Therapies
Emerging evidence suggests that mitochondria-targeted therapies acting upstream of cellular damage could present advantages over targeting downstream processes, such as inflammation and fibrosis. Novel therapeutic strategies for mitochondrial disease are being developed in preclinical studies and are entering human clinical trials [128,129,130]. Mitochondria-targeted therapies aim to enhance mitochondrial function, acting on electron transfer chain (ETC) function, mitochondrial biogenesis or promoting FAO, or to dampen the cellular consequences of mitochondrial dysfunction, including ROS production, inflammasome activation, apoptosis, pyroptosis, and autophagy/mitophagy. Treatments that enhance ETC components, and therefore electron transfer (CoQ10, idebenone, and riboflavin) or increase ETC substrate availability (dichloroacetate, and thiamine) have been tested in mitochondrial diseases [128]. Mitochondria-targeted therapies were nephroprotective in experimental AKI [19]. Initial approaches used mitochondrial permeability transition pore inhibitors [19]. More recent strategies are aimed at restoring mitochondrial metabolism, redox state, and dynamics, as well as enhancing biogenesis [131,132].
Mitochondrial-targeted antioxidants, including the Szeto–Schiller (SS) peptides, MitoQ, and plastoquinone analogues (SkQ1/SkQR1), accumulate within the mitochondrial matrix and interact with cardiolipin, a major constituent of the mitochondrial inner membrane [133]. SS peptides promote ATP synthesis, reduce electron leak and ROS production, and inhibit cardiolipin peroxidation [134], preventing the consequences of mitochondrial dysfunction, including apoptosis, inflammation, and NLRP3 inflammasome activation [134]. Specifically, SSP3 protected from experimental AKI [135] and in kidney injury in T1DM induced by streptozotocin in rats [136]. MitoQ, MitoTEMPO, or SkQR1 also reduced oxidative damage and renal inflammation [134,137,138,139]. MitoQ protected from diabetes-induced CKD in T2DM db/db and in T1DM Ins2±AkitaJ mice however, in the latter model, mitochondrial function was reported to be normal [138,140].
Finally, miRNAs targeting fibrosis-associated genes (like miR21 or miR9) were protective by inducing metabolic reprograming mainly through modulation of mitochondrial-damage related genes [141,142].
5. PGC-1α in Kidney Diseases
Evidence for the contribution of decreased PGC-1α levels or activity to the pathogenesis of AKI is derived from in vivo animal studies. The role of PGC-1α in experimental kidney diseases has been explored in mice which overexpressed PGC-1α or had a PGC-1α genetic deficiency. PGC-1α-KO mice had normal renal function assessed by serum creatinine [28,94] although there was evidence of subclinical kidney injury characterized by tubulointerstitial inflammation, increased NGAL expression and oversensitivity to AKI [28]. Additionally, there is indirect evidence based on the use of PGC-1α activators for a protective role of PGC-1α in experimental CKD.
5.1. PGC-1α in AKI
There is functional in vivo evidence of the role of insufficient PGC-1α activation or even PGC-1α downregulation in the pathogenesis of diverse forms of AKI, as well as of the therapeutic impact of preserving PGC-1α expression in experimental AKI (Table 1, Figure 3A). Tubular cells are key cell types in AKI and PGC-1α was protective in these cells (Figure 3B).
Table 1.
Preclinical studies of PGC-1α modulation in AKI.
| Model | Treatment | Effect on PGC-1αand Mitochondria | Effect on Kidney Injury | Reference | |
|---|---|---|---|---|---|
| Folic acid | Anti-TWEAK antibodies | ↑ PGC-1α ↑ MB |
↑ renal function ↓ inflammation |
[37] | |
| Crotonate | ↑ PGC-1α | ↑ renal function ↓ inflammation |
[145] | ||
| Selective cGMP-specific PDE inhibitors | ↑ PGC-1α ↑ mitochondrial function ↑ MB |
↑ renal function | [34] | ||
| PGC-1α-KO mice | No PGC-1α ↓↓ MB |
↓↓ renal function ↑↑ inflammation ↑↑ tubular cell death |
[28] | ||
| NMN | ↑ Sirt1 ↑ mitochondrial function |
↑ renal function | [29] | ||
| Cisplatin | 5-Aminolevulinic acid (even better with Fe) | ↑ PGC-1α ↑ mitochondrial function |
↑ renal function ↓ tubular cell death |
[153] | |
| PDK inhibitor DCA / PDK4-KO mice | ↑ PGC-1α ↑ mitochondrial function ↑ MB |
↑ renal function ↓ tubular cell death |
[154] | ||
| AICAR / ALCAR | ↑ PGC-1α ↑ mitochondrial function ↓mitochondrial fragmentation, ↓ DRP-1 |
↑ renal function | [155] | ||
| Sepsis | TLR4-KO / Pharmacologic inhibition of MEK/ERK signaling | ↑ PGC-1α ↑ mitochondrial function ↑ MB |
↑ renal function | [162] | |
| STAC (SRT1720) | ↑ Sirt1 | ↑ renal function ↓ inflammation |
[167] | ||
| Inducible tubular transgenic mice (iNephPGC1α) | ↑ PGC-1α | ↑ renal function | [31] | ||
| IRI | NAM | ↑ PGC-1α | ↑ renal function ↓ fatty acid accumulation |
[31] | |
| Pharmacologic inhibition of MEK/ERK signaling | ↑ PGC-1α ↑ MB |
↑ renal function | [174] | ||
| STAC (SRT1720) | ↑ PGC-1α ↑ mitochondrial function ↑ MB |
↑ renal function ↓ inflammation↓ tubular cell death |
[175] | ||
| Caloric restriction | ↑ PGC-1α | ↑ renal function ↓ inflammation |
[176] | ||
| Formoterol | Unchanged PGC-1α ↑ mitochondrial function |
↑ renal function | [171] | ||
| PGC-1α-KO mice | Absent PGC-1α | ↓↓ renal function ↓↓ mitochondrial function ↑ fatty acid accumulation |
[31] | ||
| Inducible tubular transgenic mice (iNephPGC1α) | ↑ PGC-1α | ↑ renal function ↓ fatty acid accumulation |
[31] | ||
Abbreviations: AICAR: 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside, ALCAR: antioxidant agent acetyl-l-carnitine, cGMP: cyclic guanosine monophosphate, DCA: dichloroacetate, DRP-1: dynamin-related protein-1, ERK: extracellular signal-regulated kinase, MB: mitochondrial biogenesis, MEK: mitogen-activated protein kinase kinase, NAM: niacinamide, NMN: nicotinamide mononucleotide, PDE: phosphodiesterase, PDK: pyruvate dehydrogenase kinase, Sirt1: sirtuin 1, STAC: sirtuin activating compound, TLR4: toll-like receptor 4, TWEAK: TNF-like weak inducer of apoptosis.
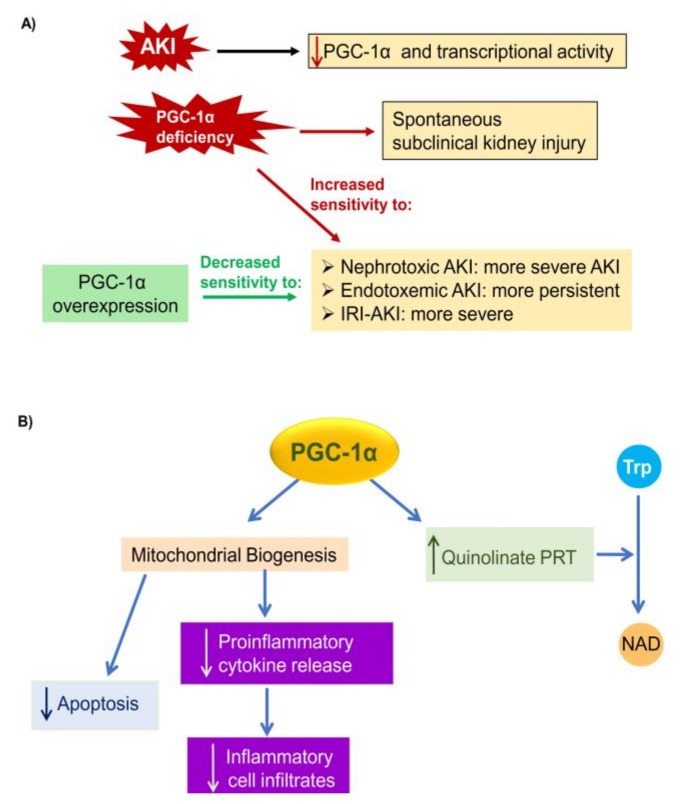
Figure 3.
PGC-1α and AKI. (A) AKI is characterized by a decrease in PGC-1α gene expression and activity leading to downregulation of PGC-1α target genes. Genetic PGC-1α deficiency is associated with spontaneous evidence of subclinical CKD as well as with increased sensitivity to AKI (more severe and/or more persistent as shown in the figure). PGC-1α overexpression in tubular cells is associated with decreased severity of experimental AKI. Additionally, interventions that result in increased PGC-1α gene transcription or activity are also associated with decreased AKI severity. (B) Nephroprotective actions of PGC-1α in tubular cells. Pathways are shown that are responsive to higher PGC-1α levels in tubular cells and/or AKI, and to contribute to nephroprotection. Abbreviations: Trp: tryptophan, NAD: nicotinamide adenine dinucleotide.
Nephrotoxic AKI. The role of PGC-1α in nephrotoxic AKI has been studied in experimental folic acid-induced AKI (FA-AKI) and cisplatin nephrotoxicity. PGC-1α mRNA expression and protein levels decrease within 24h in FA-AKI and upstream regulator analysis of kidney transcriptomic data identified PGC-1α as the transcriptional regulator whose activity is most dramatically reduced in AKI [28,37,143]. The repression of PGC-1α activity appeared to be functionally relevant given the downregulated gene expression of PGC-1α canonical targets such as electron transport chain components and TFAM leading to reduced mitochondrial biogenesis. Proinflammatory cytokines such as TWEAK were identified as key drivers of PGC-1α downregulation during AKI. In FA-AKI, neutralizing anti-TWEAK antibodies prevented the kidney downregulation of PGC-1α and its targets. In addition, systemic TWEAK administration decreased PGC-1α expression in healthy kidneys and in cultured tubular cells this decrease was prevented by NF-κB inhibitors [37]. Crotonylation, a post-translational modification of histones [144], may also regulate PGC-1α expression. Thus, crotonate, a precursor of the substrate for histone crotonylases, reduced renal injury and increased PGC-1α expression in FA-AKI [145]. However, it is unclear whether modulation of PGC-1α expression was the only or even the main driver of the nephroprotection offered by crotonate.
PGC-1α overexpression and genetic depletion approaches further support its role in nephrotoxic AKI. In cultured tubular cells, adenoviral-mediated PGC-1α overexpression prevented TWEAK-induced downregulation of PGC-1α-dependent genes and the decrease in mitochondrial membrane potential [37]. Indeed, over-expression of PGC-1α in renal proximal tubule cells restored mitochondrial and cellular functions after oxidant exposure, demonstrating the role of mitochondrial biogenesis in recovery from cellular injury [146].
PGC-1α-KO mice with FA-AKI had increased mortality and more severe loss of renal function and tubulointerstitial injury (tubular cell death and compensatory proliferation, expression of pro-inflammatory cytokines, NF-κB activation and interstitial inflammatory cell infiltration). This was associated with decreased kidney expression of mitochondrial PGC-1α-dependent genes and an earlier decrease in mitochondrial mass than in wild type (WT) mice. Thus, PGC-1α KO mice had lower mitochondrial biogenesis. The more severe inflammation in PGC-1α KO mice was characterized by increased M1 and decreased M2 responses and lower expression of the anti-inflammatory cytokine IL-10 [28]. In cultured renal tubular cells, PGC-1α targeting promoted cell death and pro-inflammatory responses [28].
Selective inhibitors of cGMP-specific PDE increased PGC-1α expression and mitochondrial biogenesis in cultured renal tubular cells, in healthy kidney and in kidneys with FA-AKI [34]. However, their effect on renal function and renal injury was not well characterized.
Nephrotoxicity is the dose-limiting adverse effect of cisplatin and interventional studies suggest that mitochondrial injury may play a critical role, as different strategies to preserve mitochondrial function were nephroprotective [106,147,148,149,150,151]. Indeed, cisplatin downregulates kidney tubular PGC-1α in vivo and in cultured tubular cells [152,153,154]. Pyruvate dehydrogenase kinase 4 (PDK4) plays a key role in cisplatin-induced AKI, since PDK4 deficiency prevented AKI and the decrease in PGC-1α expression and mitochondrial biogenesis [154]. The AMPK activator 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) and the antioxidant agent acetyl-l-carnitine (ALCAR), activate SIRT3, another member of the sirtuin family of NAD-dependent deacetylases. Both drugs increased PGC-1α expression and decreased mitochondrial fragmentation and renal function in experimental cisplatin-induced AKI, while SIRT3 deficiency increased AKI severity [155]. This fits well with the positive feedback loop between PGC-1α and SIRT3 expression [145,156]. As commented above, PGC-1α regulates NAD biosynthesis. Interestingly, the NAD precursor nicotinamide mononucleotide (NMN) increased NAD biosynthesis and protected from cisplatin nephrotoxicity and this was linked to SIRT1 [29,31,157]. However, as PGC-1α is a SIRT1 target, we cannot discard that PGC-1α modulation is required for NAD nephroprotection.
Sepsis-associated AKI (s-AKI). s-AKI is frequent in critically ill sepsis patients and is associated with a high mortality [158,159]. Experimental models reproduce s-AKI by cecal puncture ligation (a bacteremia model closer to the clinical situation) or by administering bacterial lipopolysaccharide (LPS), inducing endotoxemia and a sterile systemic inflammatory response.
s-AKI is characterized by tubular cell death, interstitial inflammatory cell infiltration and mitochondrial dysfunction [160]. Thus, swollen mitochondria were observed in human and experimental s-AKI tubules [94,161,162]. During sepsis, kidney oxygen consumption is reduced leading to decreased intracellular ATP levels, but this is not due to a reduced tissue oxygenation [94,163]. Rather, in murine LPS-induced AKI, kidney mitochondrial dysfunction, and reduced expression of PGC-1α and its mitochondrial target genes correlated with AKI severity [94,162]. As it was the case for FA-AKI, inflammatory cytokines are thought to decrease kidney PGC-1α in s-AKI, as TNF-α decreased PGC-1α expression in cultured renal tubular cells, leading to mitochondrial dysfunction, and also decreased kidney PGC-1α upon systemic administration in vivo [162]. Additionally, LPS itself directly activates TLR4 to downregulate PGC-1α via engagement of the transcription factor NF-κB in cultured cells [37,164,165], but whether NF-κB targeting in vivo preserves PGC-1α expression in s-AKI was not explored in detail. However, the role of MAPK was characterized and the MEK1/2 (MAP2K1/MAP2K2) inhibitor GSK1120212 prevented renal injury and PGC-1α downregulation following LPS injection [162]. This is consistent with prior data showing that ERK1/2 activation regulated PGC-1α expression in tissues such as brain and skeletal muscle [165,166].
Tubule-specific PGC-1α-knockout mice suffered persistent kidney injury following endotoxemia [94]. By contrast, enforced PGC-1α expression in human tubular proximal epithelial cells prevented TNF-induced mitochondrial injury, but the in vivo impact on kidney injury was not studied [94]. During sepsis, mechanisms that might restore PGC-1α expression are also activated, such as AMPK and sirtuins [167]. An improved understanding of these compensatory mechanisms may help design therapeutic approaches to preserve PGC-1α levels during s-AKI. PGC-1α expression and mitochondrial biogenesis could also be regulated by miRNAs, at least in adipocytes and skeletal muscle [168,169]. Differentially expressed serum miRNAs from s-AKI patients targeted oxidative stress and mitochondrial functions, and miR-4270, a PGC-1α regulator, was upregulated in serum of s-AKI patients [170]. This opens a new window to preserve PGC-1α expression but further studies are necessary to define the exact role of specific miRNAs in s-AKI and in mitochondrial dysfunction.
Ischemia-reperfusion injury. Kidney IRI is a frequent cause of AKI after major surgery and following kidney transplantation that is characterized by PGC-1α downregulation and mitochondrial dysfunction [31,171,172,173,174,175,176]. Kidney PGC-1α is reduced within 24 hours of kidney IRI, and PGC-1α-deficient mice had more severe AKI and accumulated fatty acids in tubular cells. This was ascribed to PGC-1α regulation of the expression of enzymes required for de novo NAD synthesis from tryptophan, and more specifically, of quinolinate phosphoribosyl transferase [30,31]. Thus, two NAD precursors (niacinamide (NAM) and NMN) were nephroprotective in murine IRI, and a phase 1 pilot study of NAM administration to patients undergoing cardiac surgery showed promising results on AKI incidence [29,30,31].
By contrast, specific tubular PGC-1α overexpression increased survival after kidney IRI, and reduced tubular injury and fatty acid accumulation [31]. This suggests that preservation of kidney PGC-1α may be protective in kidney IRI as well as for s-AKI. In this regard, strategies that increase PGC-1α expression were protective in kidney IRI. Both caloric restriction and SRT1720, which activate AMPK and Sirt1 respectively, activated PGC-1α and were protective in rat IRI-AKI and decreased kidney inflammation [175,176]. Further studies are needed to test if nephroprotection led to increased PGC-1α expression or was a consequence of increased PGC-1α [175,176]. In this regard, formoterol, a β2-adrenergic receptor agonist, decreased the severity of IRI-AKI and increased the expression of mitochondrial proteins but had a weak effect on PGC-1α expression.
5.2. PGC-1α in CKD
The role of PGC-1α in CKD and potential therapeutic approaches to preserve PGC-1α activity has been most extensively studied in diabetic nephropathy, although additional forms of CKD characterized by kidney fibrosis have also been studied.
Diabetic nephropathy. Given that diabetic nephropathy is proteinuric and characterized by mesangial extracellular matrix deposition, most studies have explored podocytes and mesangial cells, although it is likely that tubular cells are even more compromised from a mitochondrial point of view. However, the contribution of mitochondrial derangement and, specifically, podocyte PGC1α deficiency to podocyte physiology has been questioned [177]. Indeed, while PGC-1α overexpression may be beneficial in cultured tubular cells, forced PGC-1α overexpression in podocytes caused collapsing glomerulopathy, suggesting that a tight regulation in at least some cell types is required [178]. In any case, the study of diabetic nephropathy has identified PGC-1α regulators of potential therapeutic interest. Thus, PGC-1α activators were nephroprotective in both in experimental T1DM and T2DM.
Glomerular SIRT1 expression is reduced in human diabetic glomeruli; the podocyte-specific loss of SIRT1 increased albuminuria and accelerated kidney disease progression in T1DMc OVE26 mice. The selective SIRT1 agonist BT175 increased SIRT1-mediated activation of PGC1-α and protected against high glucose-mediated mitochondrial injury in cultured podocytes while both podocyte-specific SIRT1 overexpression and BT175 decreased diabetes-induced podocytopenia and glomerular oxidative stress in mice [179]. Resveratrol, a natural polyphenolic antioxidant and SIRT1 agonist, restored renal SIRT1 and PGC-1α levels in both T2DM db/db mice and in mice with T1DM induced by streptozotocin [180,181]. Resveratrol also increased PGC-1α expression and decreased apoptosis and mitochondrial oxidative stress in podocytes exposed to high glucose [180,181]. In addition, in db/db mice, inhibition of TLR4/NF-κB signaling prevented the decreased kidney PGC-1α expression, mitochondrial dysfunction and deformation, and ROS accumulation, while PGC-1α overexpression prevented mitochondrial dysfunction and cell death in cultured human proximal tubular cells in a diabetic milieu [182].
INT-767 activates the nuclear hormone receptors, farnesoid X receptor (FXR) and the G protein-coupled receptor TGR5 to confer nephroprotection in mice with streptozotocin–induced T1DM and in T2DM db/db mice (lower proteinuria, podocyte injury, mesangial expansion, and tubulointerstitial fibrosis) through recruitment of multiple pathways, including stimulation of AMPK/SIRT1/PGC-1α [183]. By contrast, the selective FXR agonist obeticholic acid, which is in clinical use for primary biliary cholangitis, did not modulate PGC-1α [183]. Human recombinant Extracellular Superoxide Dismutase (EC-SOD) also activated AMPK/PGC-1α and it reduced albuminuria, mesangial expansion, and interstitial fibrosis in db/db mice [184].
In podocytes from streptozotocin-induced T1DM mice, progranulin maintained mitochondrial homeostasis via SIRT1-PGC-1α-mediated mitochondrial biogenesis and mitophagy [185]. Pyruvate kinase M2 (PKM2) activity was decreased and the PKM2 small-molecule activator TEPP-46 reversed hyperglycemia-induced mitochondrial dysfunction, partially by increasing PGC-1α mRNA in cultured podocytes and in streptozotocin-induced diabetic mice, in whom it reduced albuminuria and diabetic glomerular histological features [186]. In cultured mesangial cells, fenofibrate improved lipotoxicity via activation of AMPK-PGC-1α [187]. Rap1b expression decreased in tubules from human diabetic nephropathy and overexpression of constitutively active Rap1b improved renal tubular mitochondrial dysfunction, oxidative stress, and apoptosis in rats with streptozotocin-induced diabetes, in association with increased C/EBP-β and PGC-1α expression [188].
PGC-1α is functionally regulated by the lncRNA taurine-upregulated gene 1 (Tug1). Podocyte-specific overexpression of Tug1 in T2DM db/db mice rescued PGC-1α expression and that of its transcriptional targets and improved mitochondrial bioenergetics and the biochemical and histological features of diabetic nephropathy. Tug1 interferes with the expression of C/EBP homologous protein (CHOP), an inhibitor of PGC-1α expression and promotes PGC-1α binding to its own promoter [189,190].
Other forms of CKD. In kidneys from CKD patients, PGC-1α and PGC-1α-dependent mitochondrial gene expression was downregulated and their expression positively correlated with the glomerular filtration rate and negatively with fibrosis [39]. Fibrosis is a key process in CKD progression, and PGC-1α was downregulated at the mRNA and protein levels in different murine of renal fibrosis, such as Notch transgenic mice and folic acid-induced kidney fibrosis. Indeed, tubule-specific overexpression of PGC-1α reduced fibrosis and restored mitochondrial content in folic acid-induced fibrosis and in Notch transgenic mice [39,121]. In line with in vivo data, TGF-β1 reduced PGC-1α mRNA in Smad3-dependent manner primary human tubular cells [39]. PGC-1α downregulation consistently leads to lipid accumulation and impaired FAO. Indeed, in human and folic acid-induced kidney fibrosis, decreased PGC-1α expression correlated with impaired FAO. FAO inhibition itself has been linked to kidney fibrosis. Thus, pharmacological or TGF-β1-induced inhibition of FAO leads to a profibrotic phenotypic shift characterized by epithelial-mesenchymal transition. This was independent from glucose metabolism, highlighting that FAO itself is a driver of fibrosis [121].
Aldosterone is a strong contributor to podocyte injury and CKD progression, and also induced mitochondrial dysfunction and PGC1a downregulation in podocytes in vivo and in vitro [191]. In fact, both, PGC-1α overexpression or preservation of PGC-1α expression by resveratrol reduced mitochondrial dysfunction and podocyte injury in aldosterone exposed mice [191,192].
6. Summary and Future Perspectives
In conclusion, there is increasing evidence for a role of an absolute or relative PGC-1α deficiency on the kidney susceptibility to diverse forms of acute and chronic injury. Indeed, low PGC-1α activity appears to be a common feature of AKI and CKD. The functional impact of PGC-1α has been conclusively demonstrated in preclinical kidney injury as PGC-1α deficiency in either whole kidney or specific kidney cell types, was deleterious while genetic PGC-1α overexpression was generally protective. However, this therapeutic approach is unlikely to reach the clinic. Thus, of even more interest is the characterization of drivers of PGC-1α downregulation that can be targeted therapeutically, including PDE, Notch1, TGF-β1 and TWEAK. Additionally, a host of drugs increases PGC-1α through diverse mechanisms, often through engagement of sirtuins. Advances in the biology and targeting of these pathways are potential avenues to develop PGC-1α activators for clinical use [193,194,195]. Nevertheless, excessive PGC-1α activation may alter mitochondrial homeostasis as observed in podocytes [178], suggesting that it is important to establish the optimal therapeutic window for PGC-1α activation. In addition, PGC-1α over activation could be deleterious in cancer [196,197], thus therapeutic strategies to preserve PGC-1α function should ideally be specific of the tissue/s in which PGC-1α downregulation is pathogenic.
Funding
Supported by ISCIII-FIS, FEDER funds, CP14/00133, PI16/02057, PI16/01900, PI18/01366, PI19/00588, PI19/00815, DTS18/00032, ERA-PerMed-JTC2018 (KIDNEY ATTACK AC18/00064 and PERSTIGAN AC18/00071, ISCIII-RETIC REDinREN RD016/0009, Sociedad Española de Nefrología, Fundacion Renal IñigoÁlvarez de Toledo (FRIAT), ISCIII Miguel Servet (A.B.S., M.D.S.-N.), ISCIII Sara Borrell (J.M.M.-M.), Comunidad de Madrid CIFRA2 B2017/BMD-3686 (M.F.-B. and D.M.-S.). No financial conflict of interest exists.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lameire N.H., Bagga A., Cruz D., De Maeseneer J., Endre Z., Kellum J.A., Liu K.D., Mehta R.L., Pannu N., Van Biesen W., et al. Acute kidney injury: An increasing global concern. Lancet. 2013;382:170–179. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 2.Iavecchia L., Cereza García G., Sabaté Gallego M., Vidal Guitart X., Ramos Terrades N., de la Torre J., Segarra Medrano A., Agustí Escasany A. Drug-related acute renal failure in hospitalised patients. Nefrologia. 2015;35:523–532. doi: 10.1016/j.nefro.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigo E., Suberviola B., Albines Z., Castellanos Á., Heras M., Rodriguez-Borregán J.C., Piñera C., Serrano M., Arias M. A comparison of acute kidney injury classification systems in sepsis. Nefrologia. 2016;36:530–534. doi: 10.1016/j.nefro.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012;2:1–138. [Google Scholar]
- 5.Martin-Sanchez D., Ruiz-Andres O., Poveda J., Carrasco S., Cannata-Ortiz P., Sanchez-Niño M.D., Ruiz Ortega M., Egido J., Linkermann A., Ortiz A., et al. Ferroptosis, but Not Necroptosis, Is Important in Nephrotoxic Folic Acid-Induced AKI. J. Am. Soc. Nephrol. 2017;28:218–229. doi: 10.1681/ASN.2015121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin-Sanchez D., Fontecha-Barriuso M., Carrasco S., Sanchez-Niño M.D., Mässenhausen A.V., Linkermann A., Cannata-Ortiz P., Ruiz-Ortega M., Egido J., Ortiz A., et al. TWEAK and RIPK1 mediate a second wave of cell death during AKI. Proc. Natl. Acad. Sci. USA. 2018;115:4182–4187. doi: 10.1073/pnas.1716578115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Von Mässenhausen A., Tonnus W., Linkermann A. Cell Death Pathways Drive Necroinflammation during Acute Kidney Injury. Nephron. 2018;140:144–147. doi: 10.1159/000490807. [DOI] [PubMed] [Google Scholar]
- 8.Martin-Sanchez D., Poveda J., Fontecha-Barriuso M., Ruiz-Andres O., Sanchez-Niño M.D., Ruiz-Ortega M., Ortiz A., Sanz A.B. Targeting of regulated necrosis in kidney disease. Nefrologia. 2018;38:125–135. doi: 10.1016/j.nefro.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Sanz A.B., Santamaría B., Ruiz-Ortega M., Egido J., Ortiz A. Mechanisms of renal apoptosis in health and disease. J. Am. Soc. Nephrol. 2008;19:1634–1642. doi: 10.1681/ASN.2007121336. [DOI] [PubMed] [Google Scholar]
- 10.Foreman K.J., Marquez N., Dolgert A., Fukutaki K., Fullman N., McGaughey M., Pletcher M.A., Smith A.E., Tang K., Yuan C.W., et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392:2052–2090. doi: 10.1016/S0140-6736(18)31694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortiz A., Sanchez-Niño M.D., Crespo-Barrio M., De-Sequera-Ortiz P., Fernández-Giráldez E., García-Maset R., Macía-Heras M., Pérez-Fontán M., Rodríguez-Portillo M., Salgueira-Lazo M., et al. The Spanish Society of Nephrology (SENEFRO) commentary to the Spain GBD 2016 report: Keeping chronic kidney disease out of sight of health authorities will only magnify the problem. Nefrologia. 2019;39:29–34. doi: 10.1016/j.nefro.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Inter. 2013;3:1–150. [Google Scholar]
- 13.Perez-Gomez M.V., Bartsch L.A., Castillo-Rodriguez E., Fernandez-Prado R., Fernandez-Fernandez B., Martin-Cleary C., Gracia-Iguacel C., Ortiz A. Clarifying the concept of chronic kidney disease for non-nephrologists. Clin. Kidney J. 2019;12:258–261. doi: 10.1093/ckj/sfz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webster A.C., Nagler E.V., Morton R.L., Masson P. Chronic Kidney Disease. Lancet. 2017;389:1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 15.Hsu R.K., Hsu C.Y. The Role of Acute Kidney Injury in Chronic Kidney Disease. Semin. Nephrol. 2016;36:283–292. doi: 10.1016/j.semnephrol.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venkatachalam M.A., Weinberg J.M., Kriz W., Bidani A.K. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J. Am. Soc. Nephrol. 2015;26:1765–1776. doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linkermann A., Chen G., Dong G., Kunzendorf U., Krautwald S., Dong Z. Regulated cell death in AKI. J. Am. Soc. Nephrol. 2014;25:2689–2701. doi: 10.1681/ASN.2014030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honarpisheh M., Desai J., Marschner J.A., Weidenbusch M., Lech M., Vielhauer V., Anders H.J., Mulay S.R. Regulated necrosis-related molecule mRNA expression in humans and mice and in murine acute tissue injury and systemic autoimmunity leading to progressive organ damage, and progressive fibrosis. Biosci. Rep. 2016;36:e00425. doi: 10.1042/BSR20160336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tábara L.C., Poveda J., Martin-Cleary C., Selgas R., Ortiz A., Sanchez-Niño M.D. Mitochondria-targeted therapies for acute kidney injury. Expert Rev. Mol. Med. 2014;16:e13. doi: 10.1017/erm.2014.14. [DOI] [PubMed] [Google Scholar]
- 20.Venkatachalam M.A., Weinberg J.M. The tubule pathology of septic acute kidney injury: A neglected area of research comes of age. Kidney Int. 2012;81:338–340. doi: 10.1038/ki.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishimoto Y., Inagi R. Mitochondria: A therapeutic target in acute kidney injury. Nephrol. Dial. Transplant. 2016;31:1062–1069. doi: 10.1093/ndt/gfv317. [DOI] [PubMed] [Google Scholar]
- 22.Uittenbogaard M., Chiaramello A. Mitochondrial biogenesis: A therapeutic target for neurodevelopmental disorders and neurodegenerative diseases. Curr. Pharm. Des. 2014;20:5574–5593. doi: 10.2174/1381612820666140305224906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li P.A., Hou X., Hao S. Mitochondrial biogenesis in neurodegeneration. J. Neurosci. Res. 2017;95:2025–2029. doi: 10.1002/jnr.24042. [DOI] [PubMed] [Google Scholar]
- 24.Villena J.A. New insights into PGC-1 coactivators: Redefining their role in the regulation of mitochondrial function and beyond. FEBS J. 2015;282:647–672. doi: 10.1111/febs.13175. [DOI] [PubMed] [Google Scholar]
- 25.Valiño-Rivas L., Cuarental L., Nuñez G., Sanz A.B., Ortiz A., Sanchez-Niño M.D. Loss of NLRP6 expression increases the severity of acute kidney injury. Nephrol. Dial. Transplant. 2019 doi: 10.1093/ndt/gfz169. [DOI] [PubMed] [Google Scholar]
- 26.Valiño-Rivas L., Cuarental L., Agustin M., Husi H., Cannata-Ortiz P., Sanz A.B., Mischak H., Ortiz A., Sanchez-Niño M.D. MAGE genes in the kidney: Identification of MAGED2 as upregulated during kidney injury and in stressed tubular cells. Nephrol. Dial. Transplant. 2019;34:1498–1507. doi: 10.1093/ndt/gfy367. [DOI] [PubMed] [Google Scholar]
- 27.Poveda J., Sanz A.B., Carrasco S., Ruiz-Ortega M., Cannata-Ortiz P., Sanchez-Niño M.D., Ortiz A. Bcl3: A regulator of NF-κB inducible by TWEAK in acute kidney injury with anti-inflammatory and antiapoptotic properties in tubular cells. Exp. Mol. Med. 2017;49:e352. doi: 10.1038/emm.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontecha-Barriuso M., Martín-Sánchez D., Martinez-Moreno J.M., Carrasco S., Ruiz-Andrés O., Monsalve M., Sanchez-Ramos C., Gómez M.J., Ruiz-Ortega M., Sánchez-Niño M.D., et al. PGC-1α deficiency causes spontaneous kidney inflammation and increases the severity of nephrotoxic AKI. J. Pathol. 2019;249:65–78. doi: 10.1002/path.5282. [DOI] [PubMed] [Google Scholar]
- 29.Guan Y., Wang S.R., Huang X.Z., Xie Q.H., Xu Y.Y., Shang D., Hao C.M. Nicotinamide Mononucleotide, an NAD. J. Am. Soc. Nephrol. 2017;28:2337–2352. doi: 10.1681/ASN.2016040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poyan Mehr A., Tran M.T., Ralto K.M., Leaf D.E., Washco V., Messmer J., Lerner A., Kher A., Kim S.H., Khoury C.C., et al. De novo NAD. Nat. Med. 2018;24:1351–1359. doi: 10.1038/s41591-018-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran M.T., Zsengeller Z.K., Berg A.H., Khankin E.V., Bhasin M.K., Kim W., Clish C.B., Stillman I.E., Karumanchi S.A., Rhee E.P., et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016;531:528–532. doi: 10.1038/nature17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finck B.N., Kelly D.P. PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J. Clin. Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitaker R.M., Corum D., Beeson C.C., Schnellmann R.G. Mitochondrial Biogenesis as a Pharmacological Target: A New Approach to Acute and Chronic Diseases. Ann. Rev. Pharmacol. Toxicol. 2016;56:229–249. doi: 10.1146/annurev-pharmtox-010715-103155. [DOI] [PubMed] [Google Scholar]
- 34.Whitaker R.M., Wills L.P., Stallons L.J., Schnellmann R.G. cGMP-selective phosphodiesterase inhibitors stimulate mitochondrial biogenesis and promote recovery from acute kidney injury. J. Pharmacol. Exp. Ther. 2013;347:626–634. doi: 10.1124/jpet.113.208017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li N., Yuan Y., Li S., Zeng C., Yu W., Shen M., Zhang R., Li C., Zhang Y., Wang H. PDE5 inhibitors protect against post-infarction heart failure. Front. Biosci. 2016;21:1194–1210. doi: 10.2741/4450. [DOI] [PubMed] [Google Scholar]
- 36.Miyashita K., Itoh H., Tsujimoto H., Tamura N., Fukunaga Y., Sone M., Yamahara K., Taura D., Inuzuka M., Sonoyama T., et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58:2880–2892. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz-Andres O., Suarez-Alvarez B., Sánchez-Ramos C., Monsalve M., Sanchez-Niño M.D., Ruiz-Ortega M., Egido J., Ortiz A., Sanz A.B. The inflammatory cytokine TWEAK decreases PGC-1α expression and mitochondrial function in acute kidney injury. Kidney Int. 2016;89:399–410. doi: 10.1038/ki.2015.332. [DOI] [PubMed] [Google Scholar]
- 38.Hindi S.M., Mishra V., Bhatnagar S., Tajrishi M.M., Ogura Y., Yan Z., Burkly L.C., Zheng T.S., Kumar A. Regulatory circuitry of TWEAK-Fn14 system and PGC-1α in skeletal muscle atrophy program. FASEB J. 2014;28:1398–1411. doi: 10.1096/fj.13-242123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han S.H., Wu M.Y., Nam B.Y., Park J.T., Yoo T.H., Kang S.W., Park J., Chinga F., Li S.Y., Susztak K. PGC-1α Protects from Notch-Induced Kidney Fibrosis Development. J. Am. Soc. Nephrol. 2017;28:3312–3322. doi: 10.1681/ASN.2017020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li S.Y., Susztak K. The Role of Peroxisome Proliferator-Activated Receptor γ Coactivator 1α (PGC-1α) in Kidney Disease. Semin. Nephrol. 2018;38:121–126. doi: 10.1016/j.semnephrol.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herzig S., Long F., Jhala U.S., Hedrick S., Quinn R., Bauer A., Rudolph D., Schutz G., Yoon C., Puigserver P., et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 42.Rhee J., Inoue Y., Yoon J.C., Puigserver P., Fan M., Gonzalez F.J., Spiegelman B.M. Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): Requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proc. Natl. Acad. Sci. USA. 2003;100:4012–4017. doi: 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koo S.H., Satoh H., Herzig S., Lee C.H., Hedrick S., Kulkarni R., Evans R.M., Olefsky J., Montminy M. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat. Med. 2004;10:530–534. doi: 10.1038/nm1044. [DOI] [PubMed] [Google Scholar]
- 44.Wu H., Deng X., Shi Y., Su Y., Wei J., Duan H. PGC-1α, glucose metabolism and type 2 diabetes mellitus. J. Endocrinol. 2016;229:R99–R115. doi: 10.1530/JOE-16-0021. [DOI] [PubMed] [Google Scholar]
- 45.Yoon J.C., Xu G., Deeney J.T., Yang S.N., Rhee J., Puigserver P., Levens A.R., Yang R., Zhang C.Y., Lowell B.B., et al. Suppression of beta cell energy metabolism and insulin release by PGC-1alpha. Dev. Cell. 2003;5:73–83. doi: 10.1016/S1534-5807(03)00170-9. [DOI] [PubMed] [Google Scholar]
- 46.Oh Y.S., Jun H.S. Role of bioactive food components in diabetes prevention: Effects on Beta-cell function and preservation. Nutr. Metab. Insights. 2014;7:51–59. doi: 10.4137/NMI.S13589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Souza C.T., Gasparetti A.L., Pereira-da-Silva M., Araújo E.P., Carvalheira J.B., Saad M.J., Boschero A.C., Carneiro E.M., Velloso L.A. Peroxisome proliferator-activated receptor gamma coactivator-1-dependent uncoupling protein-2 expression in pancreatic islets of rats: A novel pathway for neural control of insulin secretion. Diabetologia. 2003;46:1522–1531. doi: 10.1007/s00125-003-1222-5. [DOI] [PubMed] [Google Scholar]
- 48.Sun L.L., Jiang B.G., Li W.T., Zou J.J., Shi Y.Q., Liu Z.M. MicroRNA-15a positively regulates insulin synthesis by inhibiting uncoupling protein-2 expression. Diabetes Res. Clin. Pract. 2011;91:94–100. doi: 10.1016/j.diabres.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Kim J.W., Sun C., Jeon S.Y., You Y.H., Shin J.Y., Lee S.H., Cho J.H., Park C.G., Yoon K.H. Glucocorticoid treatment independently affects expansion and transdifferentiation of porcine neonatal pancreas cell clusters. BMB Rep. 2012;45:51–56. doi: 10.5483/BMBRep.2012.45.1.51. [DOI] [PubMed] [Google Scholar]
- 50.Kim J.W., Park S.Y., You Y.H., Ham D.S., Park H.S., Lee S.H., Yang H.K., Yoon K.H. Targeting PGC-1α to overcome the harmful effects of glucocorticoids in porcine neonatal pancreas cell clusters. Transplantation. 2014;97:273–279. doi: 10.1097/01.TP.0000438627.68225.25. [DOI] [PubMed] [Google Scholar]
- 51.Vandenbeek R., Khan N.P., Estall J.L. Linking Metabolic Disease With the PGC-1α Gly482Ser Polymorphism. Endocrinology. 2018;159:853–865. doi: 10.1210/en.2017-00872. [DOI] [PubMed] [Google Scholar]
- 52.Ling C., Del Guerra S., Lupi R., Rönn T., Granhall C., Luthman H., Masiello P., Marchetti P., Groop L., Del Prato S. Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion. Diabetologia. 2008;51:615–622. doi: 10.1007/s00125-007-0916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goyenechea E., Crujeiras A.B., Abete I., Parra D., Martínez J.A. Enhanced short-term improvement of insulin response to a low-caloric diet in obese carriers the Gly482Ser variant of the PGC-1alpha gene. Diabetes Res. Clin. Pract. 2008;82:190–196. doi: 10.1016/j.diabres.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 54.Zhang P., Liu C., Zhang C., Zhang Y., Shen P., Zhang J., Zhang C.Y. Free fatty acids increase PGC-1alpha expression in isolated rat islets. FEBS Lett. 2005;579:1446–1452. doi: 10.1016/j.febslet.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 55.He T.T., Cao X.P., Chen R.Z., Zhu X.N., Wang X.L., Li Y.B., Xiao H.P. Down-regulation of peroxisome proliferator-activated receptor γ coactivator-1α expression in fatty acid-induced pancreatic beta-cell apoptosis involves nuclear factor-κB pathway. Chin. Med. J. 2011;124:3657–3663. [PubMed] [Google Scholar]
- 56.Oropeza D., Jouvet N., Bouyakdan K., Perron G., Ringuette L.J., Philipson L.H., Kiss R.S., Poitout V., Alquier T., Estall J.L. PGC-1 coactivators in β-cells regulate lipid metabolism and are essential for insulin secretion coupled to fatty acids. Mol. Metab. 2015;4:811–822. doi: 10.1016/j.molmet.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kramer A., Pippias M., Noordzij M., Stel V.S., Andrusev A.M., Aparicio-Madre M.I., Arribas Monzón F.E., Åsberg A., Barbullushi M., Beltrán P., et al. The European Renal Association - European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2016: A summary. Clin. Kidney J. 2019;12:702–720. doi: 10.1093/ckj/sfz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma K., Karl B., Mathew A.V., Gangoiti J.A., Wassel C.L., Saito R., Pu M., Sharma S., You Y.H., Wang L., et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J. Am. Soc. Nephrol. 2013;24:1901–1912. doi: 10.1681/ASN.2013020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li L., Wang C., Yang H., Liu S., Lu Y., Fu P., Liu J. Metabolomics reveal mitochondrial and fatty acid metabolism disorders that contribute to the development of DKD in T2DM patients. Mol. Biosyst. 2017;13:2392–2400. doi: 10.1039/C7MB00167C. [DOI] [PubMed] [Google Scholar]
- 60.Pérez S., Rius-Pérez S., Finamor I., Martí-Andrés P., Prieto I., García R., Monsalve M., Sastre J. Obesity causes PGC-1α deficiency in the pancreas leading to marked IL-6 upregulation via NF-κB in acute pancreatitis. J. Pathol. 2019;247:48–59. doi: 10.1002/path.5166. [DOI] [PubMed] [Google Scholar]
- 61.Lin J., Wu P.H., Tarr P.T., Lindenberg K.S., St-Pierre J., Zhang C.Y., Mootha V.K., Jäger S., Vianna C.R., Reznick R.M., et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 62.Aharoni-Simon M., Hann-Obercyger M., Pen S., Madar Z., Tirosh O. Fatty liver is associated with impaired activity of PPARγ-coactivator 1α (PGC1α) and mitochondrial biogenesis in mice. Lab. Invest. 2011;91:1018–1028. doi: 10.1038/labinvest.2011.55. [DOI] [PubMed] [Google Scholar]
- 63.Farrell G.C., Larter C.Z. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 64.Vodkin I., Kuo A. Extended Criteria Donors in Liver Transplantation. Clin. Liver Dis. 2017;21:289–301. doi: 10.1016/j.cld.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 65.Sánchez-Ramos C., Prieto I., Tierrez A., Laso J., Valdecantos M.P., Bartrons R., Roselló-Catafau J., Monsalve M. PGC-1α Downregulation in Steatotic Liver Enhances Ischemia-Reperfusion Injury and Impairs Ischemic Preconditioning. Antioxid. Redox Signal. 2017;27:1332–1346. doi: 10.1089/ars.2016.6836. [DOI] [PubMed] [Google Scholar]
- 66.Chaung W.W., Jacob A., Ji Y., Wang P. Suppression of PGC-1alpha by Ethanol: Implications of Its Role in Alcohol Induced Liver Injury. Int. J. Clin. Exp. Med. 2008;1:161–170. [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X., Ji R., Sun H., Peng J., Ma X., Wang C., Fu Y., Bao L., Jin Y. Scutellarin ameliorates nonalcoholic fatty liver disease through the PPARγ/PGC-1α-Nrf2 pathway. Free Radic. Res. 2018;52:198–211. doi: 10.1080/10715762.2017.1422602. [DOI] [PubMed] [Google Scholar]
- 68.Yao W., Cai H., Li X., Li T., Hu L., Peng T. Endoplasmic reticulum stress links hepatitis C virus RNA replication to wild-type PGC-1α/liver-specific PGC-1α upregulation. J. Virol. 2014;88:8361–8374. doi: 10.1128/JVI.01202-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan M., Tang C., Zhang Y., Cheng Y., Cai L., Chen X., Gao Y., Deng Y., Pan M. SIRT1/PGC-1α signaling protects hepatocytes against mitochondrial oxidative stress induced by bile acids. Free Radic. Res. 2015;49:935–945. doi: 10.3109/10715762.2015.1016020. [DOI] [PubMed] [Google Scholar]
- 70.Wang S., Wan T., Ye M., Qiu Y., Pei L., Jiang R., Pang N., Huang Y., Liang B., Ling W., et al. Nicotinamide riboside attenuates alcohol induced liver injuries via activation of SirT1/PGC-1α/mitochondrial biosynthesis pathway. Redox Biol. 2018;17:89–98. doi: 10.1016/j.redox.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim M.S., Sweeney T.R., Shigenaga J.K., Chui L.G., Moser A., Grunfeld C., Feingold K.R. Tumor necrosis factor and interleukin 1 decrease RXRalpha, PPARalpha, PPARgamma, LXRalpha, and the coactivators SRC-1, PGC-1alpha, and PGC-1beta in liver cells. Metabolism. 2007;56:267–279. doi: 10.1016/j.metabol.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Speckmann B., Walter P.L., Alili L., Reinehr R., Sies H., Klotz L.O., Steinbrenner H. Selenoprotein P expression is controlled through interaction of the coactivator PGC-1alpha with FoxO1a and hepatocyte nuclear factor 4alpha transcription factors. Hepatology. 2008;48:1998–2006. doi: 10.1002/hep.22526. [DOI] [PubMed] [Google Scholar]
- 73.Lopes Junior E., Leite H.P., Konstantyner T. Selenium and selenoproteins: From endothelial cytoprotection to clinical outcomes. Transl. Res. 2019;208:85–104. doi: 10.1016/j.trsl.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 74.Felder T.K., Soyal S.M., Oberkofler H., Hahne P., Auer S., Weiss R., Gadermaier G., Miller K., Krempler F., Esterbauer H., et al. Characterization of novel peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) isoform in human liver. J. Biol. Chem. 2011;286:42923–42936. doi: 10.1074/jbc.M111.227496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Byrne C.D., Targher G. NAFLD as a driver of chronic kidney disease. J. Hepatol. 2020 doi: 10.1016/j.jhep.2020.01.013. in press. [DOI] [PubMed] [Google Scholar]
- 76.Wattacheril J. Extrahepatic Manifestations of Nonalcoholic Fatty Liver Disease. Gastroenterol. Clin. 2020;49:141–149. doi: 10.1016/j.gtc.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 77.Valle I., Alvarez-Barrientos A., Arza E., Lamas S., Monsalve M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 2005;66:562–573. doi: 10.1016/j.cardiores.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 78.Borniquel S., Valle I., Cadenas S., Lamas S., Monsalve M. Nitric oxide regulates mitochondrial oxidative stress protection via the transcriptional coactivator PGC-1alpha. FASEB J. 2006;20:1889–1891. doi: 10.1096/fj.05-5189fje. [DOI] [PubMed] [Google Scholar]
- 79.Sun H., Zhu X., Zhou Y., Cai W., Qiu L. C1q/TNF-Related Protein-9 Ameliorates Ox-LDL-Induced Endothelial Dysfunction via PGC-1α/AMPK-Mediated Antioxidant Enzyme Induction. Int. J. Mol. Sci. 2017;18:e1097. doi: 10.3390/ijms18061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.García-Quintans N., Prieto I., Sánchez-Ramos C., Luque A., Arza E., Olmos Y., Monsalve M. Regulation of endothelial dynamics by PGC-1α relies on ROS control of VEGF-A signaling. Free Radic. Biol. Med. 2016;93:41–51. doi: 10.1016/j.freeradbiomed.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 81.Borniquel S., García-Quintáns N., Valle I., Olmos Y., Wild B., Martínez-Granero F., Soria E., Lamas S., Monsalve M. Inactivation of Foxo3a and subsequent downregulation of PGC-1 alpha mediate nitric oxide-induced endothelial cell migration. Mol. Cell. Biol. 2010;30:4035–4044. doi: 10.1128/MCB.00175-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.García-Quintans N., Sánchez-Ramos C., Prieto I., Tierrez A., Arza E., Alfranca A., Redondo J.M., Monsalve M. Oxidative stress induces loss of pericyte coverage and vascular instability in PGC-1α-deficient mice. Angiogenesis. 2016;19:217–228. doi: 10.1007/s10456-016-9502-0. [DOI] [PubMed] [Google Scholar]
- 83.Zhang M., Chu Y., Mowery J., Konkel B., Galli S., Theos A.C., Golestaneh N. Pgc-1α repression and high-fat diet induce age-related macular degeneration-like phenotypes in mice. Dis. Model. Mech. 2018;11:dmm032698. doi: 10.1242/dmm.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Whaley-Connell A., Sowers J.R. Obesity and kidney disease: From population to basic science and the search for new therapeutic targets. Kidney Int. 2017;92:313–323. doi: 10.1016/j.kint.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 85.Zannad F., Rossignol P. Cardiorenal Syndrome Revisited. Circulation. 2018;138:929–944. doi: 10.1161/CIRCULATIONAHA.117.028814. [DOI] [PubMed] [Google Scholar]
- 86.Vanholder R., Fouque D., Glorieux G., Heine G.H., Kanbay M., Mallamaci F., Massy Z.A., Ortiz A., Rossignol P., Wiecek A., et al. Clinical management of the uraemic syndrome in chronic kidney disease. Lancet Diabetes Endocrinol. 2016;4:360–373. doi: 10.1016/S2213-8587(16)00033-4. [DOI] [PubMed] [Google Scholar]
- 87.Zoccali C., Vanholder R., Massy Z.A., Ortiz A., Sarafidis P., Dekker F.W., Fliser D., Fouque D., Heine G.H., Jager K.J., et al. The systemic nature of CKD. Nat. Rev. Nephrol. 2017;13:344–358. doi: 10.1038/nrneph.2017.52. [DOI] [PubMed] [Google Scholar]
- 88.Soltoff S.P. ATP and the regulation of renal cell function. Ann. Rev. Physiol. 1986;48:9–31. doi: 10.1146/annurev.ph.48.030186.000301. [DOI] [PubMed] [Google Scholar]
- 89.Weidemann M.J., Krebs H.A. The fuel of respiration of rat kidney cortex. Biochem. J. 1969;112:149–166. doi: 10.1042/bj1120149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang C., Youle R.J. The role of mitochondria in apoptosis*. Ann. Rev. Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roy M., Reddy P.H., Iijima M., Sesaki H. Mitochondrial division and fusion in metabolism. Curr. Opin. Cell. Biol. 2015;33:111–118. doi: 10.1016/j.ceb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brooks C., Wei Q., Cho S.G., Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Howell G.M., Gomez H., Collage R.D., Loughran P., Zhang X., Escobar D.A., Billiar T.R., Zuckerbraun B.S., Rosengart M.R. Augmenting autophagy to treat acute kidney injury during endotoxemia in mice. PLoS ONE. 2013;8:e69520. doi: 10.1371/journal.pone.0069520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tran M., Tam D., Bardia A., Bhasin M., Rowe G.C., Kher A., Zsengeller Z.K., Akhavan-Sharif M.R., Khankin E.V., Saintgeniez M., et al. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J. Clin. Invest. 2011;121:4003–4014. doi: 10.1172/JCI58662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Galloway C.A., Lee H., Nejjar S., Jhun B.S., Yu T., Hsu W., Yoon Y. Transgenic control of mitochondrial fission induces mitochondrial uncoupling and relieves diabetic oxidative stress. Diabetes. 2012;61:2093–2104. doi: 10.2337/db11-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhan M., Usman I.M., Sun L., Kanwar Y.S. Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J. Am. Soc. Nephrol. 2015;26:1304–1321. doi: 10.1681/ASN.2014050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bartz R.R., Fu P., Suliman H.B., Crowley S.D., MacGarvey N.C., Welty-Wolf K., Piantadosi C.A. Staphylococcus aureus sepsis induces early renal mitochondrial DNA repair and mitochondrial biogenesis in mice. PLoS ONE. 2014;9:e100912. doi: 10.1371/journal.pone.0100912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parikh S.M., Yang Y., He L., Tang C., Zhan M., Dong Z. Mitochondrial function and disturbances in the septic kidney. Semin. Nephrol. 2015;35:108–119. doi: 10.1016/j.semnephrol.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jankauskas S.S., Andrianova N.V., Alieva I.B., Prusov A.N., Matsievsky D.D., Zorova L.D., Pevzner I.B., Savchenko E.S., Pirogov Y.A., Silachev D.N., et al. Dysfunction of Kidney Endothelium after Ischemia/Reperfusion and Its Prevention by Mitochondria-Targeted Antioxidant. Biochemistry. 2016;81:1538–1548. doi: 10.1134/S0006297916120154. [DOI] [PubMed] [Google Scholar]
- 100.Wang H., Guan Y., Karamercan M.A., Ye L., Bhatti T., Becker L.B., Baur J.A., Sims C.A. Resveratrol Rescues Kidney Mitochondrial Function Following Hemorrhagic Shock. Shock. 2015;44:173–180. doi: 10.1097/SHK.0000000000000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Szeto H.H., Liu S., Soong Y., Wu D., Darrah S.F., Cheng F.Y., Zhao Z., Ganger M., Tow C.Y., Seshan S.V. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J. Am. Soc. Nephrol. 2011;22:1041–1052. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fedorova L.V., Sodhi K., Gatto-Weis C., Puri N., Hinds T.D., Shapiro J.I., Malhotra D. Peroxisome proliferator-activated receptor δ agonist, HPP593, prevents renal necrosis under chronic ischemia. PLoS ONE. 2013;8:e64436. doi: 10.1371/journal.pone.0064436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Plotnikov E.Y., Kazachenko A.V., Vyssokikh M.Y., Vasileva A.K., Tcvirkun D.V., Isaev N.K., Kirpatovsky V.I., Zorov D.B. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int. 2007;72:1493–1502. doi: 10.1038/sj.ki.5002568. [DOI] [PubMed] [Google Scholar]
- 104.Heidari R., Ahmadi A., Mohammadi H., Ommati M.M., Azarpira N., Niknahad H. Mitochondrial dysfunction and oxidative stress are involved in the mechanism of methotrexate-induced renal injury and electrolytes imbalance. Biomed. Pharmacother. 2018;107:834–840. doi: 10.1016/j.biopha.2018.08.050. [DOI] [PubMed] [Google Scholar]
- 105.Niknahad H., Heidari R., Mohammadzadeh R., Ommati M.M., Khodaei F., Azarpira N., Abdoli N., Zarei M., Asadi B., Rasti M., et al. Sulfasalazine induces mitochondrial dysfunction and renal injury. Ren. Fail. 2017;39:745–753. doi: 10.1080/0886022X.2017.1399908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mukhopadhyay P., Horváth B., Zsengellér Z., Zielonka J., Tanchian G., Holovac E., Kechrid M., Patel V., Stillman I.E., Parikh S.M., et al. Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free Radic. Biol. Med. 2012;52:497–506. doi: 10.1016/j.freeradbiomed.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Funk J.A., Schnellmann R.G. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am. J. Physiol. Renal Physiol. 2012;302:F853–F864. doi: 10.1152/ajprenal.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.López S., Negredo E., Garrabou G., Puig J., Ruiz L., Sanjurjo E., Ramos X., Infante A.B., Casademont J., Cardellach F., et al. Longitudinal study on mitochondrial effects of didanosine-tenofovir combination. AIDS Res. Hum. Retroviruses. 2006;22:33–39. doi: 10.1089/aid.2006.22.33. [DOI] [PubMed] [Google Scholar]
- 109.Yuan J., Zhou J., Chen B.C., Zhang X., Zhou H.M., Du D.F., Chang S., Chen Z.K. Magnesium supplementation prevents chronic cyclosporine nephrotoxicity via adjusting nitric oxide synthase activity. Transplant. Proc. 2005;37:1892–1895. doi: 10.1016/j.transproceed.2005.02.098. [DOI] [PubMed] [Google Scholar]
- 110.Zsengellér Z.K., Ellezian L., Brown D., Horváth B., Mukhopadhyay P., Kalyanaraman B., Parikh S.M., Karumanchi S.A., Stillman I.E., Pacher P. Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. J. Histochem. Cytochem. 2012;60:521–529. doi: 10.1369/0022155412446227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang M., Wang Y., Wang Q., Yang J., Yang D., Liu J., Li J. Involvement of mitochondria-mediated apoptosis in ethylbenzene-induced renal toxicity in rat. Toxicol. Sci. 2010;115:295–303. doi: 10.1093/toxsci/kfq046. [DOI] [PubMed] [Google Scholar]
- 112.Finsterer J. Mitochondriopathies. Eur. J. Neurol. 2004;11:163–186. doi: 10.1046/j.1351-5101.2003.00728.x. [DOI] [PubMed] [Google Scholar]
- 113.Kobayashi A., Goto Y., Nagata M., Yamaguchi Y. Granular swollen epithelial cells: A histologic and diagnostic marker for mitochondrial nephropathy. Am. J. Surg. Pathol. 2010;34:262–270. doi: 10.1097/PAS.0b013e3181cb4ed3. [DOI] [PubMed] [Google Scholar]
- 114.Kawakami T., Gomez I.G., Ren S., Hudkins K., Roach A., Alpers C.E., Shankland S.J., D’Agati V.D., Duffield J.S. Deficient Autophagy Results in Mitochondrial Dysfunction and FSGS. J. Am. Soc. Nephrol. 2015;26:1040–1052. doi: 10.1681/ASN.2013111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goto Y., Itami N., Kajii N., Tochimaru H., Endo M., Horai S. Renal tubular involvement mimicking Bartter syndrome in a patient with Kearns-Sayre syndrome. J. Pediatr. 1990;116:904–910. doi: 10.1016/S0022-3476(05)80648-1. [DOI] [PubMed] [Google Scholar]
- 116.Lake N.J., Bird M.J., Isohanni P., Paetau A. Leigh syndrome: Neuropathology and pathogenesis. J. Neuropathol. Exp. Neurol. 2015;74:482–492. doi: 10.1097/NEN.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 117.Sun L., Xie P., Wada J., Kashihara N., Liu F.Y., Zhao Y., Kumar D., Chugh S.S., Danesh F.R., Kanwar Y.S. Rap1b GTPase ameliorates glucose-induced mitochondrial dysfunction. J. Am. Soc. Nephrol. 2008;19:2293–2301. doi: 10.1681/ASN.2008030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tan A.L., Sourris K.C., Harcourt B.E., Thallas-Bonke V., Penfold S., Andrikopoulos S., Thomas M.C., O’Brien R.C., Bierhaus A., Cooper M.E., et al. Disparate effects on renal and oxidative parameters following RAGE deletion, AGE accumulation inhibition, or dietary AGE control in experimental diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2010;298:F763–F770. doi: 10.1152/ajprenal.00591.2009. [DOI] [PubMed] [Google Scholar]
- 119.Wei P.Z., Szeto C.C. Mitochondrial dysfunction in diabetic kidney disease. Clin. Chim. Acta. 2019;496:108–116. doi: 10.1016/j.cca.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 120.Zepeda-Orozco D., Kong M., Scheuermann R.H. Molecular Profile of Mitochondrial Dysfunction in Kidney Transplant Biopsies Is Associated With Poor Allograft Outcome. Transplant. Proc. 2015;47:1675–1682. doi: 10.1016/j.transproceed.2015.04.086. [DOI] [PubMed] [Google Scholar]
- 121.Kang H.M., Ahn S.H., Choi P., Ko Y.A., Han S.H., Chinga F., Park A.S., Tao J., Sharma K., Pullman J., et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015;21:37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Small D.M., Bennett N.C., Roy S., Gabrielli B.G., Johnson D.W., Gobe G.C. Oxidative stress and cell senescence combine to cause maximal renal tubular epithelial cell dysfunction and loss in an in vitro model of kidney disease. Nephron. Exp. Nephrol. 2012;122:123–130. doi: 10.1159/000350726. [DOI] [PubMed] [Google Scholar]
- 123.Cui J., Shi S., Sun X., Cai G., Cui S., Hong Q., Chen X., Bai X.Y. Mitochondrial autophagy involving renal injury and aging is modulated by caloric intake in aged rat kidneys. PLoS ONE. 2013;8:e69720. doi: 10.1371/journal.pone.0069720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mukunyadzi P., Huang H., Liu K., Fan C.Y. Concomitant loss of mitochondria and the DNA repair protein hOGG1 in clear cell carcinoma of the kidney. Appl. Immunohistochem. Mol. Morphol. 2003;11:334–338. doi: 10.1097/00129039-200312000-00010. [DOI] [PubMed] [Google Scholar]
- 125.Hervouet E., Godinot C. Mitochondrial disorders in renal tumors. Mitochondrion. 2006;6:105–117. doi: 10.1016/j.mito.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 126.Hervouet E., Simonnet H., Godinot C. Mitochondria and reactive oxygen species in renal cancer. Biochimie. 2007;89:1080–1088. doi: 10.1016/j.biochi.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 127.Meierhofer D., Mayr J.A., Foetschl U., Berger A., Fink K., Schmeller N., Hacker G.W., Hauser-Kronberger C., Kofler B., Sperl W. Decrease of mitochondrial DNA content and energy metabolism in renal cell carcinoma. Carcinogenesis. 2004;25:1005–1010. doi: 10.1093/carcin/bgh104. [DOI] [PubMed] [Google Scholar]
- 128.El-Hattab A.W., Zarante A.M., Almannai M., Scaglia F. Therapies for mitochondrial diseases and current clinical trials. Mol. Genet. Metab. 2017;122:1–9. doi: 10.1016/j.ymgme.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lagler F.B. Current and Emerging Therapies for Mitochondriopathies. Handb. Exp. Pharmacol. 2019:1–9. doi: 10.1007/164_2019_264. [DOI] [PubMed] [Google Scholar]
- 130.Hirano M., Emmanuele V., Quinzii C.M. Emerging therapies for mitochondrial diseases. Essays Biochem. 2018;62:467–481. doi: 10.1042/EBC20170114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Galvan D.L., Green N.H., Danesh F.R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017;92:1051–1057. doi: 10.1016/j.kint.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bhargava P., Schnellmann R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017;13:629–646. doi: 10.1038/nrneph.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Birk A.V., Chao W.M., Liu S., Soong Y., Szeto H.H. Disruption of cytochrome c heme coordination is responsible for mitochondrial injury during ischemia. Biochim. Biophys. Acta. 2015;1847:1075–1084. doi: 10.1016/j.bbabio.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Szeto H.H. Pharmacologic Approaches to Improve Mitochondrial Function in AKI and CKD. J. Am. Soc. Nephrol. 2017;28:2856–2865. doi: 10.1681/ASN.2017030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Szeto H.H., Liu S., Soong Y., Seshan S.V., Cohen-Gould L., Manichev V., Feldman L.C., Gustafsson T. Mitochondria Protection after Acute Ischemia Prevents Prolonged Upregulation of IL-1. J. Am. Soc. Nephrol. 2017;28:1437–1449. doi: 10.1681/ASN.2016070761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang X., Tang D., Zou Y., Wu X., Chen Y., Li H., Chen S., Shi Y., Niu H. A mitochondrial-targeted peptide ameliorated podocyte apoptosis through a HOCl-alb-enhanced and mitochondria-dependent signalling pathway in diabetic rats and in vitro. J. Enzym. Inhib. Med. Chem. 2019;34:394–404. doi: 10.1080/14756366.2018.1488697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Livingston M.J., Wang J., Zhou J., Wu G., Ganley I.G., Hill J.A., Yin X.M., Dong Z. Clearance of damaged mitochondria via mitophagy is important to the protective effect of ischemic preconditioning in kidneys. Autophagy. 2019;15:2142–2162. doi: 10.1080/15548627.2019.1615822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chacko B.K., Reily C., Srivastava A., Johnson M.S., Ye Y., Ulasova E., Agarwal A., Zinn K.R., Murphy M.P., Kalyanaraman B., et al. Prevention of diabetic nephropathy in Ins2(+/)⁻(AkitaJ) mice by the mitochondria-targeted therapy MitoQ. Biochem. J. 2010;432:9–19. doi: 10.1042/BJ20100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Patil N.K., Parajuli N., MacMillan-Crow L.A., Mayeux P.R. Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: Mitochondria-targeted antioxidant mitigates injury. Am. J. Physiol. Renal Physiol. 2014;306:F734–F743. doi: 10.1152/ajprenal.00643.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xiao L., Xu X., Zhang F., Wang M., Xu Y., Tang D., Wang J., Qin Y., Liu Y., Tang C., et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017;11:297–311. doi: 10.1016/j.redox.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chau B.N., Xin C., Hartner J., Ren S., Castano A.P., Linn G., Li J., Tran P.T., Kaimal V., Huang X., et al. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci. Transl. Med. 2012;4:121ra118. doi: 10.1126/scitranslmed.3003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fierro-Fernández M., Miguel V., Márquez-Expósito L., Nuevo-Tapioles C., Herrero J.I., Blanco-Ruiz E., Tituaña J., Castillo C., Cannata P., Monsalve M., et al. MiR-9-5p protects from kidney fibrosis by metabolic reprogramming. FASEB J. 2020;34:410–431. doi: 10.1096/fj.201901599RR. [DOI] [PubMed] [Google Scholar]
- 143.Stallons L.J., Whitaker R.M., Schnellmann R.G. Suppressed mitochondrial biogenesis in folic acid-induced acute kidney injury and early fibrosis. Toxicol. Lett. 2014;224:326–332. doi: 10.1016/j.toxlet.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fontecha-Barriuso M., Martin-Sanchez D., Ruiz-Andres O., Poveda J., Sanchez-Niño M.D., Valiño-Rivas L., Ruiz-Ortega M., Ortiz A., Sanz A.B. Targeting epigenetic DNA and histone modifications to treat kidney disease. Nephrol. Dial. Transplant. 2018;33:1875–1886. doi: 10.1093/ndt/gfy009. [DOI] [PubMed] [Google Scholar]
- 145.Ruiz-Andres O., Sanchez-Niño M.D., Cannata-Ortiz P., Ruiz-Ortega M., Egido J., Ortiz A., Sanz A.B. Histone lysine crotonylation during acute kidney injury in mice. Dis. Model. Mech. 2016;9:633–645. doi: 10.1242/dmm.024455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rasbach K.A., Schnellmann R.G. Signaling of mitochondrial biogenesis following oxidant injury. J. Biol. Chem. 2007;282:2355–2362. doi: 10.1074/jbc.M608009200. [DOI] [PubMed] [Google Scholar]
- 147.Gordon J.A., Gattone V.H. Mitochondrial alterations in cisplatin-induced acute renal failure. Am. J. Physiol. 1986;250:F991–F998. doi: 10.1152/ajprenal.1986.250.6.F991. [DOI] [PubMed] [Google Scholar]
- 148.Manohar S., Leung N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018;31:15–25. doi: 10.1007/s40620-017-0392-z. [DOI] [PubMed] [Google Scholar]
- 149.Yang Y., Liu H., Liu F., Dong Z. Mitochondrial dysregulation and protection in cisplatin nephrotoxicity. Arch. Toxicol. 2014;88:1249–1256. doi: 10.1007/s00204-014-1239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Santos N.A., Catão C.S., Martins N.M., Curti C., Bianchi M.L., Santos A.C. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch. Toxicol. 2007;81:495–504. doi: 10.1007/s00204-006-0173-2. [DOI] [PubMed] [Google Scholar]
- 151.Ahmed L.A., Shehata N.I., Abdelkader N.F., Khattab M.M. Tempol, a superoxide dismutase mimetic agent, ameliorates cisplatin-induced nephrotoxicity through alleviation of mitochondrial dysfunction in mice. PLoS ONE. 2014;9:e108889. doi: 10.1371/journal.pone.0108889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Portilla D., Dai G., McClure T., Bates L., Kurten R., Megyesi J., Price P., Li S. Alterations of PPARalpha and its coactivator PGC-1 in cisplatin-induced acute renal failure. Kidney Int. 2002;62:1208–1218. doi: 10.1111/j.1523-1755.2002.kid553.x. [DOI] [PubMed] [Google Scholar]
- 153.Terada Y., Inoue K., Matsumoto T., Ishihara M., Hamada K., Shimamura Y., Ogata K., Taniguchi Y., Horino T., Karashima T., et al. 5-Aminolevulinic acid protects against cisplatin-induced nephrotoxicity without compromising the anticancer efficiency of cisplatin in rats in vitro and in vivo. PLoS ONE. 2013;8:e80850. doi: 10.1371/journal.pone.0080850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Oh C.J., Ha C.M., Choi Y.K., Park S., Choe M.S., Jeoung N.H., Huh Y.H., Kim H.J., Kweon H.S., Lee J.M., et al. Pyruvate dehydrogenase kinase 4 deficiency attenuates cisplatin-induced acute kidney injury. Kidney Int. 2017;91:880–895. doi: 10.1016/j.kint.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 155.Morigi M., Perico L., Rota C., Longaretti L., Conti S., Rottoli D., Novelli R., Remuzzi G., Benigni A. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J. Clin. Invest. 2015;125:715–726. doi: 10.1172/JCI77632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kong X., Wang R., Xue Y., Liu X., Zhang H., Chen Y., Fang F., Chang Y. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS ONE. 2010;5:e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Oh G.S., Kim H.J., Choi J.H., Shen A., Choe S.K., Karna A., Lee S.H., Jo H.J., Yang S.H., Kwak T.H., et al. Pharmacological activation of NQO1 increases NAD+ levels and attenuates cisplatin-mediated acute kidney injury in mice. Kidney Int. 2014;85:547–560. doi: 10.1038/ki.2013.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Peerapornratana S., Manrique-Caballero C.L., Gómez H., Kellum J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96:1083–1099. doi: 10.1016/j.kint.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Poston J.T., Koyner J.L. Sepsis associated acute kidney injury. BMJ. 2019;364:k4891. doi: 10.1136/bmj.k4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lerolle N., Nochy D., Guérot E., Bruneval P., Fagon J.Y., Diehl J.L., Hill G. Histopathology of septic shock induced acute kidney injury: Apoptosis and leukocytic infiltration. Intensive Care Med. 2010;36:471–478. doi: 10.1007/s00134-009-1723-x. [DOI] [PubMed] [Google Scholar]
- 161.Takasu O., Gaut J.P., Watanabe E., To K., Fagley R.E., Sato B., Jarman S., Efimov I.R., Janks D.L., Srivastava A., et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am. J. Respir. Crit. Care Med. 2013;187:509–517. doi: 10.1164/rccm.201211-1983OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Smith J.A., Stallons L.J., Collier J.B., Chavin K.D., Schnellmann R.G. Suppression of mitochondrial biogenesis through toll-like receptor 4-dependent mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling in endotoxin-induced acute kidney injury. J. Pharmacol. Exp. Ther. 2015;352:346–357. doi: 10.1124/jpet.114.221085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sun J., Zhang J., Tian J., Virzì G.M., Digvijay K., Cueto L., Yin Y., Rosner M.H., Ronco C. Mitochondria in Sepsis-Induced AKI. J. Am. Soc. Nephrol. 2019;30:1151–1161. doi: 10.1681/ASN.2018111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shi J., Jiang B., Qiu Y., Guan J., Jain M., Cao X., Bauer M., Su L., Burkly L.C., Leone T.C., et al. PGC1α plays a critical role in TWEAK-induced cardiac dysfunction. PLoS ONE. 2013;8:e54054. doi: 10.1371/journal.pone.0054054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Coll T., Jové M., Rodríguez-Calvo R., Eyre E., Palomer X., Sánchez R.M., Merlos M., Laguna J.C., Vázquez-Carrera M. Palmitate-mediated downregulation of peroxisome proliferator-activated receptor-gamma coactivator 1alpha in skeletal muscle cells involves MEK1/2 and nuclear factor-kappaB activation. Diabetes. 2006;55:2779–2787. doi: 10.2337/db05-1494. [DOI] [PubMed] [Google Scholar]
- 166.Ashabi G., Ramin M., Azizi P., Taslimi Z., Alamdary S.Z., Haghparast A., Ansari N., Motamedi F., Khodagholi F. ERK and p38 inhibitors attenuate memory deficits and increase CREB phosphorylation and PGC-1α levels in Aβ-injected rats. Behav. Brain Res. 2012;232:165–173. doi: 10.1016/j.bbr.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 167.Khader A., Yang W.L., Hansen L.W., Rajayer S.R., Prince J.M., Nicastro J.M., Coppa G.F., Wang P. SRT1720, a sirtuin 1 activator, attenuates organ injury and inflammation in sepsis. J. Surg. Res. 2017;219:288–295. doi: 10.1016/j.jss.2017.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lemecha M., Morino K., Imamura T., Iwasaki H., Ohashi N., Ida S., Sato D., Sekine O., Ugi S., Maegawa H. MiR-494-3p regulates mitochondrial biogenesis and thermogenesis through PGC1-α signalling in beige adipocytes. Sci. Rep. 2018;8:15096. doi: 10.1038/s41598-018-33438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lima T.I., Araujo H.N., Menezes E.S., Sponton C.H., Araújo M.B., Bomfim L.H., Queiroz A.L., Passos M.A., Sousa T.A.E., Hirabara S.M. Role of microRNAs on the Regulation of Mitochondrial Biogenesis and Insulin Signaling in Skeletal Muscle. J. Cell. Physiol. 2017;232:958–966. doi: 10.1002/jcp.25645. [DOI] [PubMed] [Google Scholar]
- 170.Ge Q.M., Huang C.M., Zhu X.Y., Bian F., Pan S.M. Differentially expressed miRNAs in sepsis-induced acute kidney injury target oxidative stress and mitochondrial dysfunction pathways. PLoS ONE. 2017;12:e0173292. doi: 10.1371/journal.pone.0173292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jesinkey S.R., Funk J.A., Stallons L.J., Wills L.P., Megyesi J.K., Beeson C.C., Schnellmann R.G. Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2014;25:1157–1162. doi: 10.1681/ASN.2013090952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Baniene R., Trumbeckas D., Kincius M., Pauziene N., Raudone L., Jievaltas M., Trumbeckaite S. Short ischemia induces rat kidney mitochondria dysfunction. J. Bioenerg. Biomembr. 2016;48:77–85. doi: 10.1007/s10863-016-9643-2. [DOI] [PubMed] [Google Scholar]
- 173.Salvadori M., Rosso G., Bertoni E. Update on ischemia-reperfusion injury in kidney transplantation: Pathogenesis and treatment. World J. Transplant. 2015;5:52–67. doi: 10.5500/wjt.v5.i2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Collier J.B., Whitaker R.M., Eblen S.T., Schnellmann R.G. Rapid Renal Regulation of Peroxisome Proliferator-activated Receptor γ Coactivator-1α by Extracellular Signal-Regulated Kinase 1/2 in Physiological and Pathological Conditions. J. Biol. Chem. 2016;291:26850–26859. doi: 10.1074/jbc.M116.754762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Khader A., Yang W.L., Kuncewitch M., Jacob A., Prince J.M., Asirvatham J.R., Nicastro J., Coppa G.F., Wang P. Sirtuin 1 activation stimulates mitochondrial biogenesis and attenuates renal injury after ischemia-reperfusion. Transplantation. 2014;98:148–156. doi: 10.1097/TP.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 176.Lempiäinen J., Finckenberg P., Mervaala E.E., Sankari S., Levijoki J., Mervaala E.M. Caloric restriction ameliorates kidney ischaemia/reperfusion injury through PGC-1α-eNOS pathway and enhanced autophagy. Acta Physiol. 2013;208:410–421. doi: 10.1111/apha.12120. [DOI] [PubMed] [Google Scholar]
- 177.Brinkkoetter P.T., Bork T., Salou S., Liang W., Mizi A., Özel C., Koehler S., Hagmann H.H., Ising C., Kuczkowski A., et al. Anaerobic Glycolysis Maintains the Glomerular Filtration Barrier Independent of Mitochondrial Metabolism and Dynamics. Cell. Rep. 2019;27:1551–1566. doi: 10.1016/j.celrep.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li S.Y., Park J., Qiu C., Han S.H., Palmer M.B., Arany Z., Susztak K. Increasing the level of peroxisome proliferator-activated receptor γ coactivator-1α in podocytes results in collapsing glomerulopathy. JCI Insight. 2017;2:e92930. doi: 10.1172/jci.insight.92930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hong Q., Zhang L., Das B., Li Z., Liu B., Cai G., Chen X., Chuang P.Y., He J.C., Lee K. Increased podocyte Sirtuin-1 function attenuates diabetic kidney injury. Kidney Int. 2018;93:1330–1343. doi: 10.1016/j.kint.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang T., Chi Y., Kang Y., Lu H., Niu H., Liu W., Li Y. Resveratrol ameliorates podocyte damage in diabetic mice via SIRT1/PGC-1α mediated attenuation of mitochondrial oxidative stress. J. Cell. Physiol. 2019;234:5033–5043. doi: 10.1002/jcp.27306. [DOI] [PubMed] [Google Scholar]
- 181.Kim M.Y., Lim J.H., Youn H.H., Hong Y.A., Yang K.S., Park H.S., Chung S., Ko S.H., Koh S.H., Shin S.J., et al. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1α axis in db/db mice. Diabetologia. 2013;56:204–217. doi: 10.1007/s00125-012-2747-2. [DOI] [PubMed] [Google Scholar]
- 182.Yuan S., Liu X., Zhu X., Qu Z., Gong Z., Li J., Xiao L., Yang Y., Liu H., Sun L., et al. The Role of TLR4 on PGC-1. Oxid. Med. Cell. Longev. 2018;2018:6296802. doi: 10.1155/2018/6296802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang X.X., Wang D., Luo Y., Myakala K., Dobrinskikh E., Rosenberg A.Z., Levi J., Kopp J.B., Field A., Hill A., et al. FXR/TGR5 Dual Agonist Prevents Progression of Nephropathy in Diabetes and Obesity. J. Am. Soc. Nephrol. 2018;29:118–137. doi: 10.1681/ASN.2017020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hong Y.A., Lim J.H., Kim M.Y., Kim Y., Park H.S., Kim H.W., Choi B.S., Chang Y.S., Kim T.Y., Park C.W. Extracellular Superoxide Dismutase Attenuates Renal Oxidative Stress Through the Activation of Adenosine Monophosphate-Activated Protein Kinase in Diabetic Nephropathy. Antioxid. Redox Signal. 2018;28:1543–1561. doi: 10.1089/ars.2017.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhou D., Zhou M., Wang Z., Fu Y., Jia M., Wang X., Liu M., Zhang Y., Sun Y., Lu Y., et al. PGRN acts as a novel regulator of mitochondrial homeostasis by facilitating mitophagy and mitochondrial biogenesis to prevent podocyte injury in diabetic nephropathy. Cell. Death Dis. 2019;10:524. doi: 10.1038/s41419-019-1754-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Qi W., Keenan H.A., Li Q., Ishikado A., Kannt A., Sadowski T., Yorek M.A., Wu I.H., Lockhart S., Coppey L.J., et al. Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat. Med. 2017;23:753–762. doi: 10.1038/nm.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hong Y.A., Lim J.H., Kim M.Y., Kim T.W., Kim Y., Yang K.S., Park H.S., Choi S.R., Chung S., Kim H.W., et al. Fenofibrate improves renal lipotoxicity through activation of AMPK-PGC-1α in db/db mice. PLoS ONE. 2014;9:e96147. doi: 10.1371/journal.pone.0096147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xiao L., Zhu X., Yang S., Liu F., Zhou Z., Zhan M., Xie P., Zhang D., Li J., Song P., et al. Rap1 ameliorates renal tubular injury in diabetic nephropathy. Diabetes. 2014;63:1366–1380. doi: 10.2337/db13-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Long J., Badal S.S., Ye Z., Wang Y., Ayanga B.A., Galvan D.L., Green N.H., Chang B.H., Overbeek P.A., Danesh F.R. Long noncoding RNA Tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. J. Clin. Invest. 2016;126:4205–4218. doi: 10.1172/JCI87927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shen H., Ming Y., Xu C., Xu Y., Zhao S., Zhang Q. Deregulation of long noncoding RNA (TUG1) contributes to excessive podocytes apoptosis by activating endoplasmic reticulum stress in the development of diabetic nephropathy. J. Cell. Physiol. 2019;234:15123–15133. doi: 10.1002/jcp.28153. [DOI] [PubMed] [Google Scholar]
- 191.Yuan Y., Huang S., Wang W., Wang Y., Zhang P., Zhu C., Ding G., Liu B., Yang T., Zhang A. Activation of peroxisome proliferator-activated receptor-γ coactivator 1α ameliorates mitochondrial dysfunction and protects podocytes from aldosterone-induced injury. Kidney Int. 2012;82:771–789. doi: 10.1038/ki.2012.188. [DOI] [PubMed] [Google Scholar]
- 192.Zhao M., Yuan Y., Bai M., Ding G., Jia Z., Huang S., Zhang A. PGC-1α overexpression protects against aldosterone-induced podocyte depletion: Role of mitochondria. Oncotarget. 2016;7:12150–12162. doi: 10.18632/oncotarget.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Aquila G., Kostina A., Vieceli Dalla Sega F., Shlyakhto E., Kostareva A., Marracino L., Ferrari R., Rizzo P., Malaschicheva A. The Notch pathway: A novel therapeutic target for cardiovascular diseases? Expert Opin. Ther. Targets. 2019;23:695–710. doi: 10.1080/14728222.2019.1641198. [DOI] [PubMed] [Google Scholar]
- 194.Sanz A.B., Izquierdo M.C., Sanchez-Niño M.D., Ucero A.C., Egido J., Ruiz-Ortega M., Ramos A.M., Putterman C., Ortiz A. TWEAK and the progression of renal disease: Clinical translation. Nephrol. Dial. Transplant. 2014;29(Suppl. S1):i54–i62. doi: 10.1093/ndt/gft342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang Y., He J., Liao M., Hu M., Li W., Ouyang H., Wang X., Ye T., Zhang Y., Ouyang L. An overview of Sirtuins as potential therapeutic target: Structure, function and modulators. Eur. J. Med. Chem. 2019;161:48–77. doi: 10.1016/j.ejmech.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 196.Dan L., Wang C., Ma P., Yu Q., Gu M., Dong L., Jiang W., Pan S., Xie C., Han J., et al. PGC1α promotes cholangiocarcinoma metastasis by upregulating PDHA1 and MPC1 expression to reverse the Warburg effect. Cell. Death Dis. 2018;9:466. doi: 10.1038/s41419-018-0494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yun C.W., Lee J.H., Lee S.H. Hypoxia-induced PGC-1α Regulates Mitochondrial Function and Tumorigenesis of Colorectal Cancer Cells. Anticancer Res. 2019;39:4865–4876. doi: 10.21873/anticanres.13672. [DOI] [PubMed] [Google Scholar]